Fig. 1.
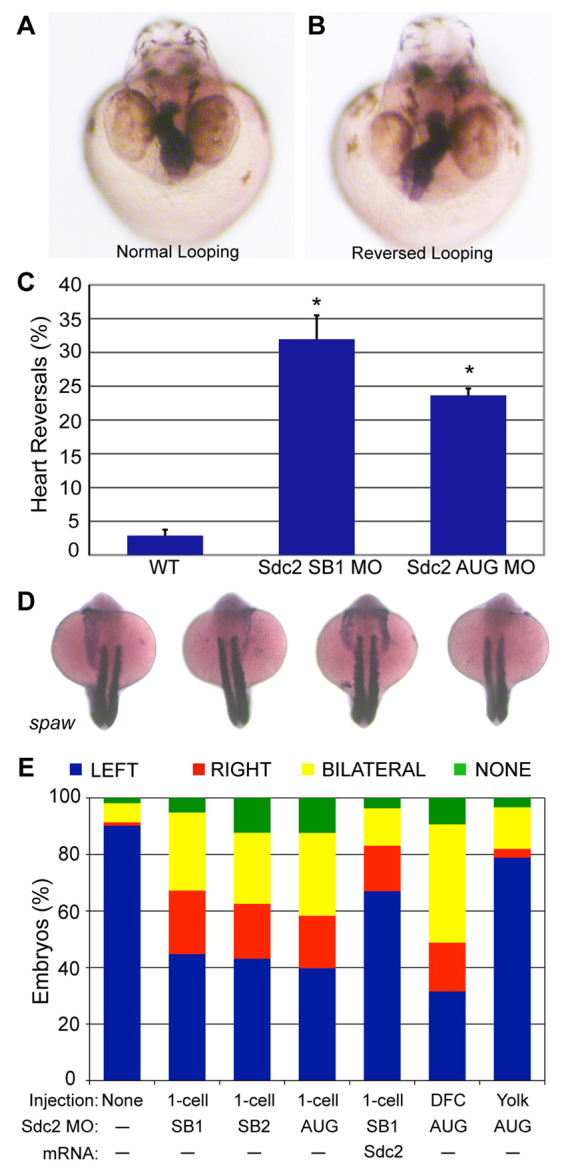
Knockdown of Sdc2 in zebrafish KV causes laterality defects. (A,B) Heart looping is visualized by labeling the heart with an RNA in situ hybridization probe for cardiac myosin light chain 2 (cmlc2) at 40 hpf. (A) Normal heart looping. (B) Reversed heart looping. (C) Both SB and AUG sdc2 morphants had a statistically significant increase in reversed heart looping (SB1 MO: 32%, n=221; AUG MO: 24%, n=369) compared with wild-type (WT) embryos (3%, n=500), *P<0.001. Error bars indicate s.e.m. (D) Examples of morphant embryos at the 18-somite stage displaying left-sided, right-sided, bilateral and absent spaw expression. Somites are labeled with myod1 which is used as a marker for staging embryo development. (E) Expression of Nodal family member spaw is mostly left-sided in wild-type embryos (n=331) but in global sdc2 morphants spaw expression is randomized (SB1 MO, n=215; SB2 MO, n=229; AUG MO, n=190; all P<0.001). Aberrant spaw expression is partially rescued with co-injection of a MO-resistant sdc2 mRNA (n=123; P<0.001 compared with morphants). Targeting MO to the DFCs (DFCsdc2MO embryos, n=147) randomized spaw expression, but targeting MO exclusively to the yolk cell did not (yolksdc2MO embryos, n=181; P<0.001 compared with global and DFC morphants). These results indicate that the role of Sdc2 in LR development is cell-autonomous within the DFCs and that extra-embryonic Sdc2 is not involved in LR development. P-values by Student’s t-test.
