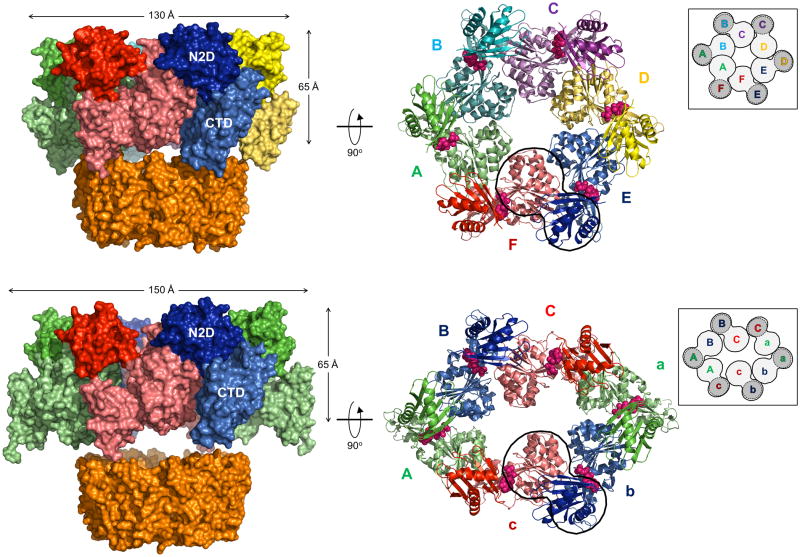
Figure 2. Crystal structures of hexameric ΔN1GspEEpsE from Vibrio cholerae fused to Hcp1.
Insets: schematic view of the hexamer outlining all six CTD•N2D’ construction units. CTD domains in light grey, N2D’ domains in dark grey.
Figure 2A. V. cholerae ΔN1GspEEpsE-6aa-Hcp1, with a quasi-C6 ΔN1GspEEpsE hexamer and V. cholerae ΔN1GspEEpsE-8aa-Hcp1 containing a C2 hexamer.
Upper panel: The quasi-C6 hexamer of V. cholerae ΔN1GspEEpsE-6aa-Hcp1.
This fusion forms a ΔN1GspEEpsE hexamer with quasi-C6 point group symmetry. Shown are subunits A (green), B (cyan), C (purple), D (yellow), E (blue) and F (red). The CTDs are shown in a lighter shade of the same color as the N2Ds of the same subunit. The Hcp1 assistant hexamer is shown in orange. Left: view perpendicular to the quasi sixfold depicting also the Hcp1 hexamer. Right: view along the quasi-sixfold axis of the ΔN1GspEEpsE hexamer, with the Hcp1 hexamer omitted. The nucleotides shown in spheres are AMPPNP superposed from the helical Δ90GspEEpsE structure (PDB: 1p9w (Robien et al., 2003)). The shape of the hexamer in this view is very regular. One CTD•N2D’ construction unit is outlined.
Lower panel: The C2 hexamer of V. cholerae ΔN1GspEEpsE-8aa-Hcp1.
This fusion forms a ΔN1GspEEpsE hexamer with C2 point group symmetry. Shown are subunits A (green), B (blue), C (red) – each occurring twice in the hexamer. The CTDs are shown in a lighter shade of the same color as the N2Ds of the same subunit. The Hcp1 assistant hexamer is shown in orange. Left: view perpendicular to the twofold depicting also the Hcp1 hexamer. Right: view along the twofold axis of the ΔN1GspEEpsE hexamer, with the Hcp1 hexamer omitted. The nucleotides shown in pink spheres are AMPPNP superposed from the helical Δ90GspEEpsE structure (PDB: 1p9w (Robien et al., 2003)). The shape of the hexamer in this view is an approximate ellipsoid of 105 Å by 150 Å. One CTD•N2D’ construction unit is outlined. See also Figure S2, S3, S4 and S7.
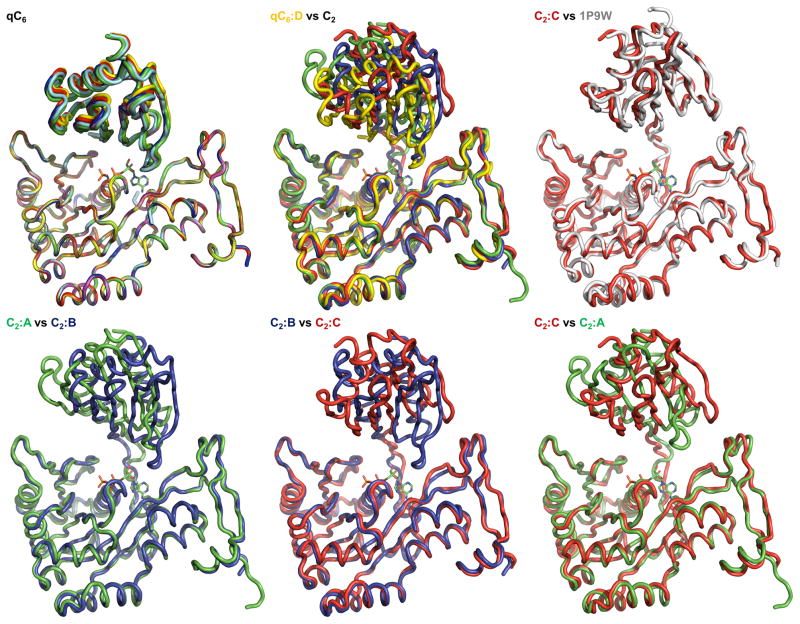
Figure 2B. The variability of the N2D-vs-CTD orientations in V. cholerae GspEEpsE.
Shown are superimposed subunits in the “canonical view” with the CTDs superimposed below and the N2Ds on top (colored as Figure 2A). For a different “orthogonal view,” see Figure 2C. The nucleotide shown for reference is AMPPNP from the helical Δ90GspEEpsE structure (PDB: 1p9w (Robien et al)). For N2D-vs-CTD orientations see also Table S1.
Top left: Superposition of the six subunits of the qC6 ΔN1GspEEpsE hexamer from the ΔN1GspEEpsE-6aa-Hcp1 structure, revealing only small differences, by one to five degrees, in N2D-vs-CTD orientations.
Top middle: Superposition of subunit D (yellow) from of the qC6 ΔN1GspEEpsE hexamer and the three subunits of the C2 ΔN1GspEEpsE hexamer from the ΔN1GspEEpsE-8aa-Hcp1 structure. N2D-vs-CTD orientations vary by 16 to 41 degrees.
Top right: Superposition of subunit C (red) from the ΔN1C2 GspEEpsE hexamer and ΔN1GspEEpsE (grey) from the helical Δ90GspEEpsE structure (PDB: 1p9w (Robien et al)). The difference in N2Dvs-CTD orientation is only 2 degrees.
Bottom left: Superposition of subunits A (green) and B (blue) of the C2 ΔN1GspEEpsE hexamer. The difference in N2D-vs-CTD orientation is 32 degrees.
Bottom middle: Superposition of subunits B (blue) and C (red) of the C2 ΔN1GspEEpsE hexamer. The difference in N2D-vs-CTD orientation is 48 degrees.
Bottom right: Superposition of subunits C (red) and A (green) of the C2 ΔN1GspEEpsE hexamer. The difference in N2D-vs-CTD orientation is 47 degrees.
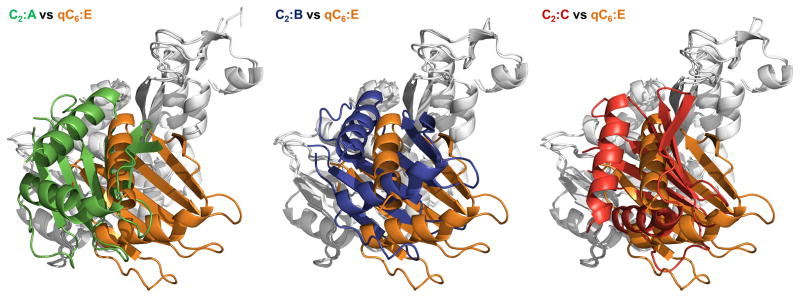
Figure 2C. “Orthogonal views” of the subunits in V. cholerae GspEEpsE.
Pairwise comparison of ΔN1GspEEpsE subunits after superposition of the CTDs (grey, as background)viewed in a direction approximately perpendicular to the “canonical view” in Figure 2B. The N2D of subunit E from the qC6 ΔN1GspEEpsE hexamer (orange) is used as reference for each case.