Abstract
Increasing evidence links the complement system with complications of human diabetes. The complement regulatory protein CD59, an inhibitor of formation of membrane attack complex (MAC), is inhibited by hyperglycemia-induced glycation fostering increased deposition of MAC, a major effector of complement-mediated tissue damage. CD59, an ubiquitous GPI-anchored membrane protein, is shed from cell membranes by phospholipases generating a soluble form present in blood and urine.
Methods
We established an ELISA to measure serum/plasma glycated hCD59 (GCD59) and evaluated its potential as a diabetes biomarker. We used a synthetic peptide strategy to generate a) a mouse monoclonal antibody to capture hCD59, b) a rabbit monoclonal antibody to detect GCD59, and c) a GCD59 surrogate for assay standardization. ELISA conditions were optimized for precision, reproducibility and clinical sensitivity. The clinical utility of the assay was initially evaluated in 24 subjects with or without diabetes and further validated in a study that included 100 subjects with and 90 subjects without a diagnosis of diabetes.
Results
GCD59 a) was significantly higher in individuals with than in individual without diabetes, b) was independently associated with HbA1c, and) identified individuals with diabetes with high specificity and sensitivity.
Conclusions
We report the development and standardization of a novel, sensitive, and specific ELISA for measuring GCD59 in blood. The assay distinguished individuals with diabetes from those without, and showed strong correlation between GCD59 and HbA1c. Because GCD59 likely contributes to the pathogenesis of diabetes complications, measurement of blood levels of GCD59 may be useful in the diagnosis and management of diabetes.
Keywords: Complement System, Membrane Attack Complex, CD59, Diabetes, Glycation, Biomarker
Introduction
Prior evidence supports a strong link between the complement system, activity of the complement regulatory protein CD59, and the pathogenesis of vascular complications of diabetes [1-6]. Complement activation ultimately leads to formation of the cytotoxic, pore-forming membrane attack complex (MAC), the main effector of complement-mediated tissue damage. Insertion of the MAC into cell membranes induces the release of cytokines and growth factors that promote inflammation, thrombosis, and cell proliferation, as characteristically seen in target organs of diabetic complications [1, 7-9].
CD59 is a complement regulatory protein ubiquitously expressed on mammalian cell surfaces; it specifically inhibits MAC formation and thereby protects “self” cells from complement-mediated damage [10, 11]. We and others have reported that the complement regulatory function of hCD59 is reduced in diabetes [1, 3, 12]; this is because 1) the non-enzymatic glycation of a Lys41 residue within the active site of hCD59 results in the formation of functionally inactive glycated CD59 (GCD59) [1, 12], and 2) there is decreased CD59 expression [3].
The co-localization of MAC deposits and GCD59 in kidneys, nerves, and vasculature of patients with diabetes further support glycation-inactivation of hCD59 as one potential mechanism contributing to the hyperglycemia-associated tissue damage responsible for the major vascular complications of human diabetes [8]. Though hCD59 is a cell membrane glycophosphatidylinositol(GPI)-bound protein, soluble hCD59 shed off from cell membranes by action of phospholipases is present in human blood (serum/plasma concentration ≈ 100 ng/ml) [13] and urine (≈ 4 ug/ml) [14]. We hypothesized that blood levels of GCD59 may represent a novel pathogenically relevant biomarker that reflects integrated blood glucose levels over time. We report here the development, optimization and validation of an ELISA for serum/plasma GCD59. Our novel methodology offers a prototypical example that can serve as a general solution to the challenging problem of assay standardization for post-translationally modified proteins, particularly those that, like GCD59, are in low abundance, heterogeneous in nature, and difficult to purify. In addition, we evaluated the clinical utility of this novel assay in 24 subjects with and without diabetes. The availability of a sensitive and specific assay to measure GCD59 will foster the conduction of large human studies to assess the potential clinical utility of GCD59 in the management of diabetes and the role of complement in the pathogenesis of the diabetes complications, a major cause of morbidity and mortality in the adult population.
Methods
To establish an ELISA to measure GCD59, we 1) developed a method to separate GCD59 from non-glycated CD59 (NGCD59), 2) synthesized hCD59-derived peptide antigens based on the amino acid sequence of hCD59 and used them to develop two monoclonal antibodies, one for capture of hCD59 (termed MABTotCD59 and recognizing all forms of hCD59) and one for detection of the GCD59 fraction in a sandwich ELISA (termed MABGlyCD59), and 3) synthesized a surrogate GCD59 that was used as a standard enabling reproducible quantification of GCD59 in large cohorts of individuals across a broad spectrum of glycemic control. Once the assay was established and optimized, we assessed its clinical utility by measuring plasma GCD59 in two groups of individuals, one with diabetes and the other without diabetes.
Purification of NGCD59 and GCD59
NGCD59 and GCD59 needed for antibody validation and assay development were separated by affinity chromatography using the anti-CD59 mouse monoclonal antibody BRIC-229 (IBGRL, UK). Like other activity neutralizing anti-CD59 antibodies [1, 12], BRIC-229 preferentially binds to and retains NGCD59, separating it from GCD59 that flows through the BRIC-229 affinity chromatography column. Since urine is a rich source of CD59 [1, 15], 50 ml of a urine pool obtained from two poorly controlled diabetic individuals was run twice through a 3 ml column in which biotinylated BRIC-229 was immobilized on streptavidine-sepharose beads (GE Healthcare, Sweden). After washes with 30 ml PBS/0.05% Tween-20/0.05% sodium azide, NGCD59 was eluted from the BRIC-229 column with 10 ml 0.1M glycine-HCl buffer, pH 3. The eluate was dialyzed 48 hours at 4°C, lyophilized and reconstituted in PBS, pH 7.4. GCD59 was purified form the flow-through fraction of the BRIC-229 column. Briefly, after dialysis and lyophilization of the BRIC-229 column flow-through, it was reconstituted in 3 ml Tris-buffered saline (TBS), pH 7.5. GCD59 was immunoprecipitated with a commercially available goat polyclonal anti-CD59 antibody (R & D Systems, USA) using protein G-agarose beads (Sigma, USA). GCD59 was eluted from the spun down protein G-agarose beads by incubation with 0.1M glycine-HCl buffer, pH 3.
Purified NGCD59 and GCD59 were separated by SDS-PAGE and transferred to PVDF membranes. All secondary antibodies used were conjugated to either IRDye700 (red fluorescence) or IRDye-800 (green fluorescence) (Rockland, USA), and signals from the immunoblots were scanned using an infrared Odyssey scanner (LI-COR Biosciences).
Anti–glycated hCD59 rabbit monoclonal antibody
A rabbit monoclonal antibody (termed MABGlyCD59) that would recognize the NaBH4 reduced post-translationally K41 glucose-modified GCD59 but not NGCD59 was raised in collaboration with Epitomics (San Diego, California) following established protocols [16]. The antigen was a 14–amino acid peptide (hCD59[37–50]) synthesized by solid-phase chemistry using a pre-formed Lys(Nε-glucitol) residue at the position corresponding to K41 in the native protein, as previously described [17]. Rabbits were immunized with this antigen peptide conjugated to KLH. Hybridoma supernatants were initially selected by ELISA screening using the synthetic glycated peptide antigen and a non-glycated peptide of identical sequence (negative control). Final selection of clones and characterization of the selected antibody was performed by WB analysis of affinity-purified GCD59 and NGCD59.
Anti-hCD59 mouse monoclonal antibody
To capture hCD59 in an ELISA assay, we raised a mouse monoclonal antibody (termed MABTotCD59) against a hCD59 epitope away from the glycation site; the antigen was a 23 amino acid peptide (hCD59[44–66]), synthesized by solid-phase chemistry and conjugated to KLH for immunization. Hybridoma supernatants were initially selected by ELISA screening against the antigen peptide, and final clones were selected by titration against recombinant human CD59 (R&D Systems, USA), following established protocols [18].
ELISA for GCD59
Capture MABTotCD59 mouse monoclonal antibody (0.3 μg/well in 50mM carbonate-bicarbonate buffer, pH 9.6 at 25°C) was coated on the wells of ELISA plates (overnight at 4°C) and then blocked with 0.25 ml protein-free blocking buffer (Thermo Scientific, USA; 1hr at room temperature). Human plasma or serum samples (50 μl) were reduced by incubation with 2.5ul of 1M freshly prepared sodium borohydride (final concentration: 50 mM, at RT for 1hr), the reaction was quenched with 1% acetic acid (1ml per 50 μl of sample) and then added to the plates at the desired dilution (in 3% protein free blocking buffer/10mM EDTA/1% NP40), and incubated for 1 hr at room temperature. GCD59 was detected with MABGlyCD59 rabbit monoclonal primary antibody (100 μl/well of a 2.5ug/ml antibody solution; at RT for 2 hours), followed by goat anti-rabbit IgG-HRP (Bethyl Laboratories, USA) secondary antibody (100 μl/well of a 14 ng/ml antibody solution; RT for 1 hour) using as substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (Thermo Scientific, USA). Plates were washed between steps with 1X PBS-0.05%Tween-20.
Assay standardization and calibration
Since limited availability of purified GCD59 precluded its extensive use as an assay calibrator, we synthesized and validated a surrogate GCD59 standard. To this end, both antigenic peptides (hCD59[44-66] and (K41(Nε-glucitol)hCD59[37-50]), respectively used to raise MABTotCD59 and MABGlyCD59 antibodies, were coupled by a PEG linker using the same functional groups on the antigens and preserving the same orientation that was used in the KLH-based immunogens described above. On one end of the PEG linker a succinimidyl ester was coupled to the ε-NH2 on the side-chain of the antigen peptide hCD59[44-66]. A maleimido moiety on the other end of the PEG linker was reacted with a thiol function on the side-chain of the C-terminal cysteinyl moiety of the antigen peptide (K41(Nε-glucitol))hCD59[37-50].
Internal controls at three concentrations (low, medium and high) of GCD59 were prepared from pooled plasma samples from individuals with diabetes, and used to accept or reject the individual runs, according to pre-specified criteria following Westgard rules [19].
Human Subjects
Partners Healthcare IRB approval was obtained for the human study described below. For preliminary assessment of the potential clinical utility of the GCD59 ELISA, we tested a “training” set of 24 consecutive plasma samples collected by the Partners' Crimson Biospecimen Repository [20] from subjects who had blood drawn for routine clinical indications that included an HbA1c measurement at either the Brigham and Women's Hospital or Massachusetts General Hospital (affiliates of Partners Healthcare). In this “training set”, that was part of a larger prospective longitudinal clinical study (which is still on-going), 10 were from individuals without and 14 from individuals with diabetes. Selection criteria included: to be considered as having diabetes, a HbA1c value >6.5% [21]; to be considered as not having diabetes, a HbA1c value <6.0% and lack of any 250.XX ICD-9 code in the medical record. Additional information on subjects' glucose and HbA1c value measured in the same blood sample separated for GCD59 measurement was also available. Collected plasma was used to measure GCD59, and the mean GCD59 for each group was compared using unpaired t-tests (data reported as means +/- standard error of means). In addition, the correlation between GCD59 and HbA1c values were quantified via Pearson correlations. The level for significance was set at α=0.05, with all reported P-values as two-tailed.
We also tested a set of plasma samples from 190 consecutive patients, 100 with and 90 without a diagnosis of Type 2 Diabetes, prospectively collected by the Partners' Crimson Biospecimen Repository [20] from individuals who had blood drawn for routine clinical indications that included a HbA1c measurement. Inclusion criteria for this study were expanded to: age 18-65 years, estimated glomerular filtration rate ≥ 60 mL/min and serum, creatinine < 1.4 mg/dL in the last two years. Patients with Type 2 Diabetes had to have an ICD diagnosis of 250.XX and at least one HbA1c that was > 6.5% in the prior two years [21]. Patients without Type 2 Diabetes could not carry an ICD diagnosis indicative of diabetes and had to have at least one HbA1c value of < 6.0% in the prior two years. Additional information on subjects' age, gender and the HbA1c value measured in the same blood sample separated for GCD59 measurement was also available. Information regarding blood glucose was not available.
Analyses were performed using multivariable linear regression with adjustments for available co-variates: age, gender, and diabetes status (yes or no). Pearson coefficients (r) were determined for correlations between normally distributed variables; the level for significance was set at α=0.05, and reported P-values as two-tailed. Data analyses were performed using SAS statistical software, v9.1.
The Partners Healthcare Institutional Review Board (IRB) reviewed and approved the human study reported here, and all animal studies were approved by the Harvard Medical School Standing Committee on Animals (IACUC; protocol #03507).
Results
Purification of NGCD59 and GCD59 by affinity chromatography
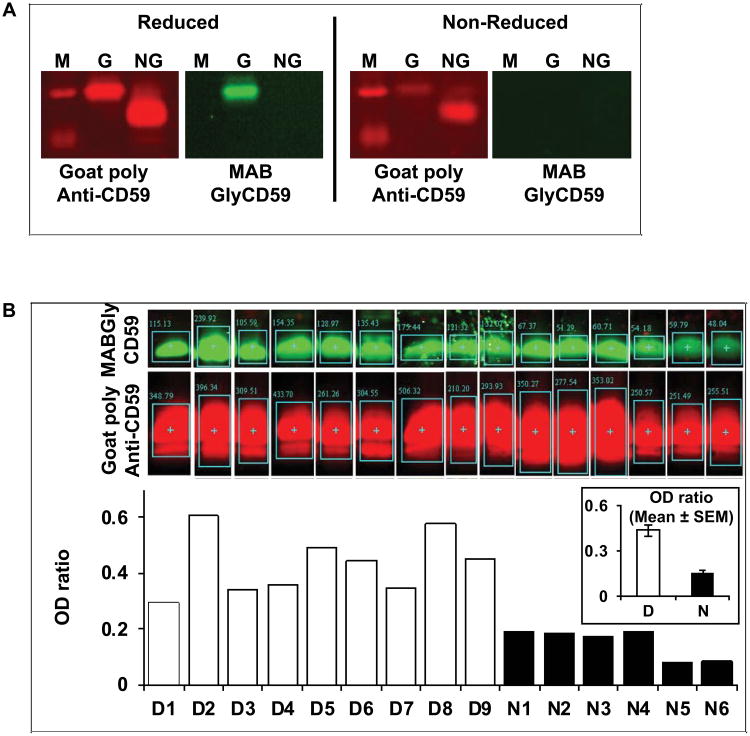
Glycation of CD59 on amino acid K41, which is located at the core of its active site [22-24], results in a parallel loss of activity and of immune reactivity towards neutralizing antibodies such as YTH53.1 and BRIC-229 [1, 12]. Preliminary experiments in our laboratory established that an affinity chromatography column that utilizes BRIC-229 binds and retains NGCD59 while GCD59 flows through the column. NGCD59 was eluted from the BRIC-229 affinity column, while GCD59 was immunoprecipitated from the column flow-through volume, as described in Methods. Figure 1A (left panels depicting red-fluorescence bands) shows the characteristic GCD59 (lane G) and NGCD59 protein bands (lane NG), purified from the urine of poorly controlled diabetic individuals and blotted with commercially available goat polyclonal anti-CD59 antibody that recognizes both NGCD59 and GCD59. Figure 1A (right panel under reduced conditions) shows that the GCD59 (but not the NGCD59) band was recognized by the MABGlyCD59 monoclonal antibody raised using the Lys41(Nε-glucitol)-modified hCD59[37–50] peptide, as described below. The full-length blotted membranes used to create this figure are shown in Supplemental Figure 1.
Figure 1. Specificity of the anti-glycated hCD59 rabbit monoclonal antibody (MABGlyCD59).
A. GCD59 (G) and NGCD59 (NG) affinity purified from a urine pool individuals with poorly controlled diabetes, separated by SDS-PAGE and immuno-blotted with goat polyclonal anti-hCD59 antibody before (non-reduced) and after (reduced) treatment with Na-borohydride (left panels in the Reduced and Non-reduced frames depicting red fluorescent bands). The GCD59 band is also recognized by the MABGlyCD59 antibody but only after Na-borohydride reduction (green fluorescent band in the G lane of the Reduced frame). The full-length blotted membranes used to create this Figure are shown in Supplemental Figure 1.
B. Affinity purified hCD59 from a group of individuals with and without diabetes separated by SDS-PAGE and immunoblotted first with goat polyclonal anti-CD59 antibody (Goat Poly Anti-CD59; red fluorescence) and then with MABGlyCD59 antibody (green fluorescence). The bar graph at the bottom of Figure 1B depicts the ratio of green (GCD59) over red (total CD59) fluorescence (quantified using an infrared Odyssey scanner) for each individual sample. The inset shows the mean ± SEM of the ratios for the individuals with (D) and without diabetes (N).
Anti–glycated hCD59 rabbit monoclonal antibody (MABGlyCD59)
The glucose-mediated glycation of proteins, a major mechanism of tissue damage in diabetes, involves the spontaneous reaction of glucose with amino groups, mostly ε-amino groups that are located at glycation motifs within specific proteins [25, 26]. The reaction is initiated by the formation of Lys(Nε-glucopyranosyl), the Schiff base, followed by a slow rearrangement to the more stable Lys(Nε-1-deoxy-fructos-1-yl), also called Amadori product [27-29]. In vitro, when the Amadori product is exposed to a reducing agent such as sodium borohydride, it is reduced to the open ring Lys(Nε-glucitol) residue [30, 31]. In the synthesis of the antigenic peptide used to raise an anti-glycated hCD59 rabbit monoclonal antibody, we introduced a pre-formed protected Lys(Nε-glucitol) residue replacing the original amino acid K41. For this reason, recognition of GCD59 by the MABGlyCD59 antibody was expected to occur only after reduction of GCD59 with sodium borohydride. This reduction converts both, Schiff base and Amadori product, into the corresponding Lys(Nε-glucitol) form. For the final round of hybridoma screening, we used WB to select hybridomas whose supernatants recognized purified GCD59 only after reduction with sodium borohydride. This method permitted exclusion of hybridomas whose supernatants reacted with NGCD59.
One of the selected and repeatedly recloned hybridomas (designated as MABGlyCD59), produced an IgG kappa 1 monoclonal antibody that recognized only the sodium borohydride reduced form of purified urine GCD59 (Figure 1A, right panels labeled with green fluorescence). Immunoblotting of the same membrane with goat anti-CD59 specific polyclonal antibody revealed that 1) the protein band detected by MABGlyCD59 was CD59 (Figure 1A, left panels under reduced and non-reduced conditions), and 2) significantly higher ratios of green (MABGlyCD59) over red (goat polyclonal anti-CD59) fluorescence were detected in samples of purified urine GCD59 from individuals with diabetes as compared to individuals without diabetes (Figure 1B).
Mouse anti-hCD59 monoclonal antibody (MABTotCD59)
Based on Bodian's et al. mapping of antibody binding sites in hCD59 [22], we designed and synthesized a series of peptide antigens corresponding to hCD59 epitopes located away from the glycation site, and used them to raise mouse monoclonal antibodies. One of these antibodies, termed MABTotCD59, showed comparable affinity towards both NGCD59 and GCD59 in WB analysis of purified protein (not shown). The specificity of the antibody for CD59 was demonstrated by WB analysis of unprocessed human urine. Urine proteins (from non-diabetic individuals) were resolved by SDS-PAGE and immunoblotted with MABTotCD59 or two commercially available monoclonal anti-hCD59 specific antibodies: BRIC-229 and goat polyclonal anti-CD59. Supplemental Figure 2 shows that MABTotCD59 recognized only one protein band in human urine and that this band is the same recognized by the other two anti-CD59 specific antibodies.
GCD59 ELISA Specificity
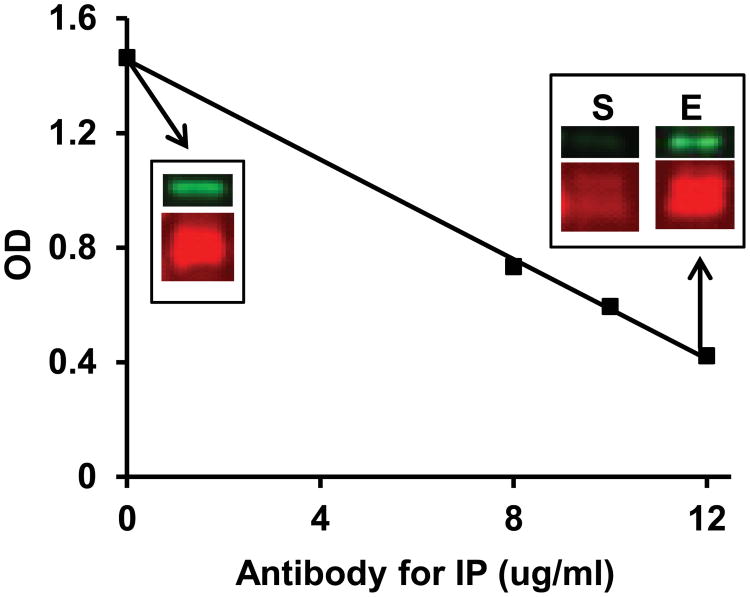
Both newly developed antibodies were used to establish a sandwich ELISA for quantitative specific measurement of serum or plasma GCD59, as described in Methods. Similar to the WB analysis shown in Figure 1A, signal in the GCD59 ELISA was elicited from either purified GCD59 or serum/plasma samples only after reduction with sodium borohydride (not shown). The specificity of the ELISA for GCD59 was confirmed by pull-down experiments in which purified GCD59 was removed by incubation with increasing concentrations of goat polyclonal anti-CD59 Ab (8, 10 and 12 μg/ml) bound to Protein-G agarose beads (Figure 2). Increasing the concentration of the anti-CD59 antibody used to pull down CD59 resulted in a linear decline in GCD59 ELISA signal in the supernatant remaining after spinning down the agarose-beads carrying CD59-anti-CD59 antibody complexes. WB analysis of the same supernatant showed a comparable progressive decline of the CD59 signal. Consistently, there was a parallel signal increase in the eluate resulting from incubation of the spun down beads carrying the CD59-Ab complexes with elution buffer (10 minutes at RT) (Figure 2). Together, these experiments indicate that the sandwich ELISA that utilizes MABTotCD59 for capture and MABGlyCD59for detection is highly specific for GCD59.
Figure 2. Specificity of the ELISA for GCD59.
Sandwich ELISA of affinity purified GCD59 before and after immunoprecipitation (IP) with increasing concentrations of goat polyclonal anti-CD59 antibody. The insets show the western blot (WB) analysis for total (red fluorescence) and GCD59 (green fluorescence) of the sample before (inset at 0 antibody concentration) and after immunopreciptiation (IP) with 12 μg/ml antibody (S= WB of the supernatant after IP; E = WB of eluate from agarose beads carrying the antibody-CD59 complex).
Sodium cyanoborohydride is a less potent and more selective reducing agent than sodium borohydride; it converts into Lys(Nε-glucitol) only the more labile Schiff base and not the Amadori product [32]. The relative contribution of Schiff base- and Amadori-modified GCD59 to the signal detected in the GCD59 ELISA was determined by pre-incubation of plasma samples from diabetic individuals with either sodium borohydride (50 mM) or sodium cyanoborohydride (75 or 100 mM; 1hr, RT). The results demonstrated that the ELISA signal elicited by GCD59 derives almost exclusively form Amadori-modified GCD59 (not shown).
Assay standardization
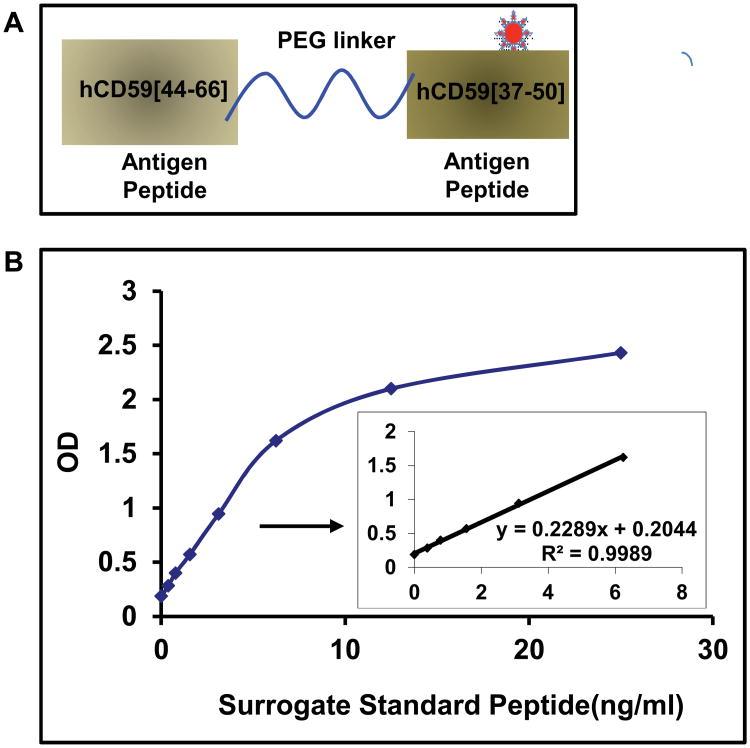
The heterogeneity and low abundance of CD59 combined with the complexity and relatively low yield of GCD59 purification prohibits the use of purified GCD59 as an ELISA calibrator for large human studies. As of today, expression of post-translationally modified proteins as well as their ab initio total synthesis are unaccomplished missions. To circumvent the challenge of GCD59 assay standardization, we synthesized a surrogate of GCD59 and used it as an assay standard and calibrator. In this surrogate, hCD59[44-66] and (K41(Nε-glucitol)hCD59[37-50], the two antigenic peptides respectively used to raise the capture (MABTotCD59) and the detection (MABGlyCD59) antibodies, were linked through a PEG linker (Figure 3A). The detailed structure of GCD59 surrogate is depicted in Supplemental Figure 3. This GCD59 surrogate was recognized by both cognate antibodies in either WB or direct ELISA (not shown) and was used to calibrate the assay. A representative calibration curve of the previously described sandwich-type ELISA that uses MABTotCD59 for capture, MABGlyCD59 for detection (primary) and GCD59 surrogate calibrator concentrations from 0.0 to 25 ng/ml is shown in Figure 3B. We observed a linear GCD59 surrogate concentration-dependent absorbance at 450 nm between 0 and 6 ng/ml (inset to Figure 3B). Levels of GCD59 in serum or plasma samples tested with the standardized GCD59 ELISA are expressed in Standard Peptide Units (SPU): one SPU is the OD reading corresponding to one ng/ml concentration of the surrogate GCD59 hybrid peptide in the calibration curve. Similar values of GCD59 were obtained when measured in paired samples of serum and plasma, an observation comparable to that of Landi [13] with an ELISA test for total CD59.
Figure 3. Synthetic Surrogate GCD59 for assay standardization.
A. Diagram of synthetic surrogate standard in which both antigen peptides, hCD59[44-66] and (K41(Nε-glucitol)hCD59[37-50] (hCD59[37-50]), respectively used to raise the capture mouse monoclonal antibody MABTotCD59 and the detection MABGlyCD59 antibodies, were coupled by a PEG linker.
 Represents the pre-formed Nε-glucitollysine residue used to synthesize the (K41(Nε-glucitol)hCD59[37-50] peptide for the position equivalent to K41 in the native protein. The detailed structure of GCD59 synthetic surrogate is shown in Supplemental Figure 3.
Represents the pre-formed Nε-glucitollysine residue used to synthesize the (K41(Nε-glucitol)hCD59[37-50] peptide for the position equivalent to K41 in the native protein. The detailed structure of GCD59 synthetic surrogate is shown in Supplemental Figure 3.
B. Characteristic surrogate GCD59 standard peptide dose-response curve in the here described sandwich ELISA that utilizes MABTotCD59 as capture and MABGlyCD59 as detection antibodies.
Basic analytical characteristics of the assay: Precision: The analysis of five determinations of the same plasma samples over a period of 5 days gave a CV <10.0% at each of the three GCD59 concentrations tested. The mean ± SD concentrations measured (and % CV) were as follows: plasma 1 (low GCD59 concentration), 0.424 ± 0.01 SPU (2.9%); plasma 2 (medium GCD59 concentration), 0.924 ± 0.08 SPU (8.3%), and plasma 3 (high GCD59 concentration), 1.72 ± 0.12 SPU (7.0%). Linearity: measurement of serially diluted plasma samples showed linearity over a GCD59 concentration range between 0.02 SPU and 4.0 SPU. Limit of detection and limit of quantification, determined by the method described in [33], were: LOD = 0.012 SPU and at 20% CV, LOQ = 0.041 SPU, respectively. Stability: GCD59 showed to be stable over a period of at least 3 years, as determined by repeated WB measurements of the same sample sub-aliquoted and stored at -80°C.
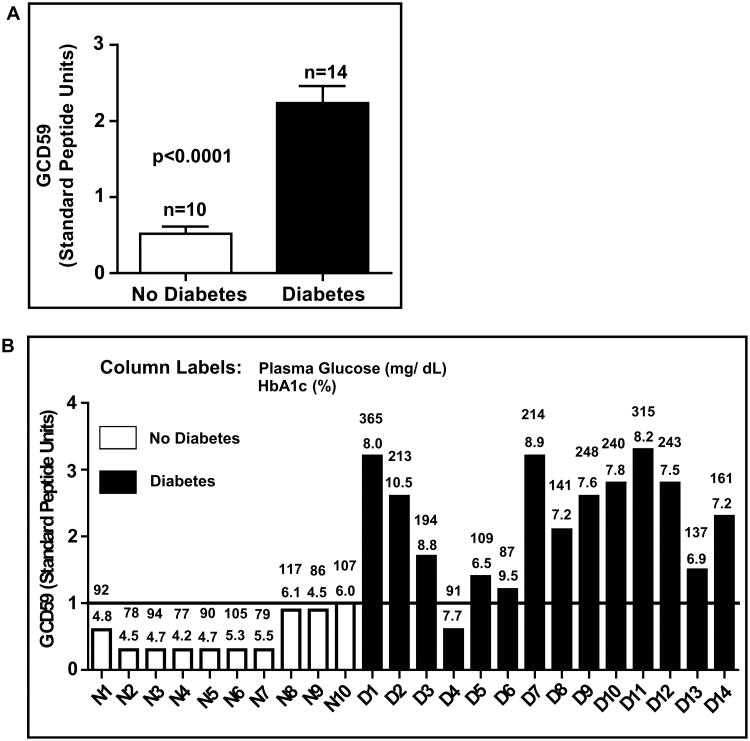
Higher GCD59 in individuals with diabetes
In the initial set of 24 samples, the ten individuals without diabetes had a mean random plasma glucose value of 92 ± 14 mg/dl and HbA1c 5.0 ± 0.6%, both significantly lower than the mean concentrations of random glucose (198 ± 84 mg/dl) and HbA1c (8.2 ± 0.99%) in the fourteen patients with diabetes (P<0.0001 and P<0.01, respectively). Figure 4A shows that mean GCD59 levels were significantly higher in individuals with diabetes than in individuals without diabetes; Figure 4B shows the individual values of GCD59 together with simultaneous values of random glucose and HbA1c. The GCD59 values were strongly associated with HbA1c (r = 0.77, Supplemental Figure 4A). The test showed a high sensitivity (93%) and specificity (100%) to discriminate diabetic form non-diabetic individuals, and generated a ROC curve with an area under the curve = 0.98 (Supplemental Figure 4B).
Figure 4. Higher levels of plasma GCD59 in individuals with as compared with individuals without diabetes.
A. Mean ± SEM of plasma GCD59 10 non-diabetic and 14 diabetic individuals.
B. Bar graph representation of the plasma GCD59 value in each study subject; the numbers above the bars represent fasting plasma glucose (top value) and HbA1c (bottom value) measured simultaneously with GCD59. In this cohort selected without any reference as to duration, treatment or control level of diabetes, a cut-off value of 1 SPU differentiates the cohorts without and with diabetes with 100 % specificity and 93% sensitivity.
In the validation human study, 100 subjects were diagnosed with T2D (HbA1c: Mean ± SEM: 9.1 ± 0.21%; age (y) Mean ± SEM: 51.8 ± 1). Samples for this cohort were collected without any reference as to duration of or specific treatments for diabetes. Another cohort of 90 subjects did not meet criteria for T2D (HbA1c: Mean ± SEM: 5.5 ± 0.05%, age (y) Mean ± SEM:: 46 ± 1). The results showed that GCD59 was significantly higher in individuals with as compared with individuals without diabetes (Mean ± SEM: no diabetes: 0.35 ± 0.03 SPU; diabetes: 1.49± 0.11 SPU, p<0.00001). The area under the curve of a Receiver Operating Characteristics (ROC) curve generated from the GCD59 values and a binary diagnosis of with or without diabetes was 0.88. In adjusted linear regression models, GCD59 concentrations were independently and positively associated with HbA1c in the entire study population (β=0.90, P<0.0001). This independent positive association between GCD59 and HbA1c was apparent when evaluated in just the subgroup of individuals with diabetes (β=0.88, P<0.001), or just the subgroup without diabetes (β=0.75, P<0.001)
Discussion
Here we describe the development and validation of a novel assay to measure circulating GCD59. The assay, which uses two specific monoclonal antibodies in a sandwich ELISA format, is highly reproducible and measures serum and/or plasma GCD59 in a highly specific and sensitive manner. Indeed, assuming a basal level of CD59 glycation of ≈ 5% (based on the basal glycation level of HbA1c in normoglycemic individuals), and that the concentration of CD59 in human serum, is ≈100-150 ng/ml [13], the reproducible detection of GCD59 in individuals without diabetes implies that the assay can detect GCD59 in the low picomolar range.
In the development of the GCD59 ELISA reported here, we designed a surrogate GCD59 strategy to generate a synthetic standard that fully recapitulates the behavior of the endogenous biomarker in an ELISA. This GCD59 surrogate can be synthesized reproducibly in large quantities allowing the performance of almost unlimited number of assays. This strategy is novel, of general applicability and provides a solution to the challenge of standardization of post-translationally modified proteins that are heterogenous, in low abundance and, therefore, not accessible through purification, ab initio total synthesis, or recombinant in vitro expression. Indeed, despite the very high abundance of hemoglobin in human blood (≈ 6 orders of magnitude higher than blood levels of CD59), a reference method for HbA1c measurements was achieved by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) only after many years of clinical use of HbA1c, and this reference method is “technically demanding, time consuming, very expensive and is not designed for routine analysis of patient samples” [34].
The clinical validation proof of concept human studies reported here showed that individuals with diabetes have significantly higher mean plasma GCD59 when compared to individuals without diabetes, that GCD59 is strongly and independently associeted with HbA1c, and that GCD59 values may be very sensitive and specific for identifying individuals with diabetes and hyperglycemia. Together, these results indicate that blood levels of GCD59 may represent a novel biomarker for glucose handling that can complement tests currently used in the diagnosis and management of diabetes, particularly HbA1c. HbA1c reflects the time-averaged glucose concentrations over a period of 6-8 weeks, the normal life span of the red blood cells that encase hemoglobin, but does not provide information about the glucose level excursions (i.e., the patient's brittleness) during that period. Even though the turnover rate of membrane-bound CD59 has not been measured in humans, it is conceivably much shorter than that of hemoglobin. This assumption is supported by preliminary data from ongoing human studies showing that upon treatment to normalize pre-prandial glucose, GCD59 parallels average weekly glucose declining to close to normal values in about two weeks (manuscript in preparation). If this is confirmed, levels of GCD59 may reflect more acute changes in glucose handling and help physicians and patients better control glucose peaks and valleys that contribute to the development of diabetic complications. A new marker such as GCD59 could also have clinical utility in instances where HbA1c is not applicable or does not perform well, including conditions that alter red cell turnover rates, some hemoglobin variants, chronic renal failure and gestational diabetes mellitus.
As a GPI-anchored membrane protein ubiquitously attached to the external surface of circulating and non-circulating cells, hCD59 is exposed to the glucose levels in the interstitial fluid, which at steady state are reportedly similar to glucose levels in venous plasma [35]. Soluble forms of CD59 present in blood and urine derive from bodily cell membranes from where GPI-linked CD59 is enzymatically shed off. [14, 36, 37]. Therefore, blood levels of soluble GCD59 likely reflect the extent of the glycation process at the cellular/tissue level. As such, GCD59 has the potential of becoming a biomarker informing on the pathogenesis of diabetes complication [1, 8]. The reasons discussed above highlight why measurements of blood levels of GCD59 made possible by the assay reported here would complement the currently available clinical tools providing meaningful information for the diagnosis, monitoring and risk stratification of patients with diabetes.
In summary, the novel GCD59 ELISA reported here permits the reproducible measurement of blood GCD59 with significant discrimination between individuals with diabetes and hyperglycemia from normoglycemic individuals. Development of this assay was a necessary condition prior to planning and conducting human studies to investigate comprehensively the clinical utility of GCD59 as a biomarker for glucose handling. Future human studies are needed to establish whether measurement of blood GCD59 may simplify the diagnosis of diabetes or impaired glucose handling, help with the assessment of integrated blood glucose values over shorter periods of time than currently available tests such as HbA1c, and the stratification of patients at risk of vascular complications of diabetes.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants DK62294 (JAH), DK089206 (JAH), and K23 HL111771-01 (AV).
Abbreviations
- MAC
membrane attack complex of complement
- hCD59
human CD59
- GCD59
glycated hCD59
- NGCD59
non-glycated hCD59
- HbA1c
glycated hemoglobin
- SPU
Standard Peptide Units
- WB
Western Blot
- KLH
keyhole limpet hemocyanin
- ELISA
enzyme-linked immunosorbent assay
- PBS
phosphate buffer saline
- RT
room temperature
Footnotes
Disclosure Statement
References
- 1.Acosta J, Hettinga J, Fluckiger R, et al. Molecular basis for a link between complement and the vascular complications of diabetes. Proc Natl Acad Sci U S A. 2000;97:5450–5455. doi: 10.1073/pnas.97.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosoklija GB, Dwork AJ, Younger DS, et al. Local activation of the complement system in endoneurial microvessels of diabetic neuropathy. Acta Neuropathol (Berl) 2000;99:55–62. doi: 10.1007/pl00007406. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Gerhardinger C, Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes. 2002;51:3499–3504. doi: 10.2337/diabetes.51.12.3499. [DOI] [PubMed] [Google Scholar]
- 4.Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol. 2010;6:94–101. doi: 10.1038/nrendo.2009.266. [DOI] [PubMed] [Google Scholar]
- 5.Mellbin LG, Bjerre M, Thiel S, et al. Complement activation and prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care. 2012;35:911–917. doi: 10.2337/dc11-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen TK, Tarnow L, Thiel S, et al. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes. 2004;53:1570–1576. doi: 10.2337/diabetes.53.6.1570. [DOI] [PubMed] [Google Scholar]
- 7.Acosta JA, Benzaquen LR, Goldstein DJ, et al. The transient pore formed by homologous terminal complement complexes functions as a bidirectional route for the transport of autocrine and paracrine signals across human cell membranes. Mol Med. 1996;2:755–765. [PMC free article] [PubMed] [Google Scholar]
- 8.Qin X, Goldfine A, Krumrei N, et al. Glycation inactivation of the complement regulatory protein CD59: a possible role in the pathogenesis of the vascular complications of human diabetes. Diabetes. 2004;53:2653–2661. doi: 10.2337/diabetes.53.10.2653. [DOI] [PubMed] [Google Scholar]
- 9.Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies A, Lachmann PJ. Membrane defence against complement lysis: the structure and biological properties of CD59. Immunol Res. 1993;12:258–275. doi: 10.1007/BF02918257. [DOI] [PubMed] [Google Scholar]
- 11.Meri S, Waldmann H, Lachmann PJ. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991;65:532–537. [PubMed] [Google Scholar]
- 12.Davies CS, Harris CL, Morgan BP. Glycation of CD59 impairs complement regulation on erythrocytes from diabetic subjects. Immunology. 2005;114:280–286. doi: 10.1111/j.1365-2567.2004.02086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landi AP, Wilson AB, Davies A, et al. Determination of CD59 protein in normal human serum by enzyme immunoassay, using octyl-glucoside detergent to release glycosyl-phosphatidylinositol-CD59 from lipid complex. Immunol Lett. 2003;90:209–213. doi: 10.1016/j.imlet.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CM, Harrison RA, Lachmann PJ, et al. Structure of a soluble, glycosylated form of the human complement regulatory protein CD59. Structure. 1994;2:185–199. doi: 10.1016/s0969-2126(00)00020-4. [DOI] [PubMed] [Google Scholar]
- 15.Lehto T, Meri S. Interactions of soluble CD59 with the terminal complement complexes. CD59 and C9 compete for a nascent epitope on C8 J Immunol. 1993;151:4941–4949. [PubMed] [Google Scholar]
- 16.Perk J, Gil-Bazo I, Chin Y, et al. Reassessment of id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-id1 antibody. Cancer Res. 2006;66:10870–10877. doi: 10.1158/0008-5472.CAN-06-2643. [DOI] [PubMed] [Google Scholar]
- 17.Cantel S, Kavishwar A, Schlimme M, et al. Glycated-CD59 antigen: exploration of synthetic approaches. Adv Exp Med Biol. 2009;611:317–318. doi: 10.1007/978-0-387-73657-0_141. [DOI] [PubMed] [Google Scholar]
- 18.Cabanas C, Sanchez-Madrid F. Monoclonal antibodies specific for leukocyte adhesion molecules. Selective protocols of immunization and screening assays for generation of blocking, activating and activation reporter antibodies. Methods Mol Biol. 1999;96:1–9. doi: 10.1385/1-59259-258-9:1. [DOI] [PubMed] [Google Scholar]
- 19.Westgard JO, Barry PL, Hunt MR, et al. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem. 1981;27:493–501. [PubMed] [Google Scholar]
- 20.Murphy S, Churchill S, Bry L, et al. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res. 2009;19:1675–1681. doi: 10.1101/gr.094615.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Executive summary: Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodian DL, Davis SJ, Morgan BP, et al. Mutational analysis of the active site and antibody epitopes of the complement-inhibitory glycoprotein, CD59. J Exp Med. 1997;185:507–516. doi: 10.1084/jem.185.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Abagyan R, Dong S, et al. Mapping the active site of CD59. J Exp Med. 1997;185:745–753. doi: 10.1084/jem.185.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Smith CA, Song H, et al. Insights into the human CD59 complement binding interface toward engineering new therapeutics. J Biol Chem. 2005;280:34073–34079. doi: 10.1074/jbc.M504922200. [DOI] [PubMed] [Google Scholar]
- 25.Monnier VM, Nagaraj RH, Portero-Otin M, et al. Structure of advanced Maillard reaction products and their pathological role. Nephrol Dial Transplant. 1996;11(5):20–26. doi: 10.1093/ndt/11.supp5.20. [DOI] [PubMed] [Google Scholar]
- 26.Johansen MB, Kiemer L, Brunak S. Analysis and prediction of mammalian protein glycation. Glycobiology. 2006;16:844–853. doi: 10.1093/glycob/cwl009. [DOI] [PubMed] [Google Scholar]
- 27.Hodge JE. The Amadori rearrangement. Adv Carbohydr Chem. 1955;10:169–205. doi: 10.1016/s0096-5332(08)60392-6. [DOI] [PubMed] [Google Scholar]
- 28.Baynes JW, Watkins NG, Fisher CI, et al. The Amadori product on protein: structure and reactions. Prog Clin Biol Res. 1989;304:43–67. [PubMed] [Google Scholar]
- 29.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Flückiger R, Strang CJ. Structural requirements for protein glycation. Protein Sci. 1995;4:186A. [Google Scholar]
- 31.Myint T, Hoshi S, Ookawara T, et al. Immunological detection of glycated proteins in normal and streptozotocin-induced diabetic rats using anti hexitol-lysine IgG. Biochim Biophys Acta. 1995;1272:73–79. doi: 10.1016/0925-4439(95)00067-e. [DOI] [PubMed] [Google Scholar]
- 32.Witztum JL, Steinbrecher UP, Fisher M, et al. Nonenzymatic glucosylation of homologous low density lipoprotein and albumin renders them immunogenic in the guinea pig. Proc Natl Acad Sci U S A. 1983;80:2757–2761. doi: 10.1073/pnas.80.9.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(1):S49–52. [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks DB. Measurement of hemoglobin A(1c): a new twist on the path to harmony. Diabetes Care. 2012;35:2674–2680. doi: 10.2337/dc12-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summers LK, Clark ML, Humphreys SM, et al. The use of microdialysis to monitor rapid changes in glucose concentration. Horm Metab Res. 1999;31:424–428. doi: 10.1055/s-2007-978767. [DOI] [PubMed] [Google Scholar]
- 36.Hakulinen J, Meri S. Shedding and enrichment of the glycolipid-anchored complement lysis inhibitor protectin (CD59) into milk fat globules. Immunology. 1995;85:495–501. [PMC free article] [PubMed] [Google Scholar]
- 37.Cocuzzi E, Szczotka LB, Brodbeck WG, et al. Tears contain the complement regulator CD59 as well as decay-accelerating factor (DAF) Clin Exp Immunol. 2001;123:188–195. doi: 10.1046/j.1365-2249.2001.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.