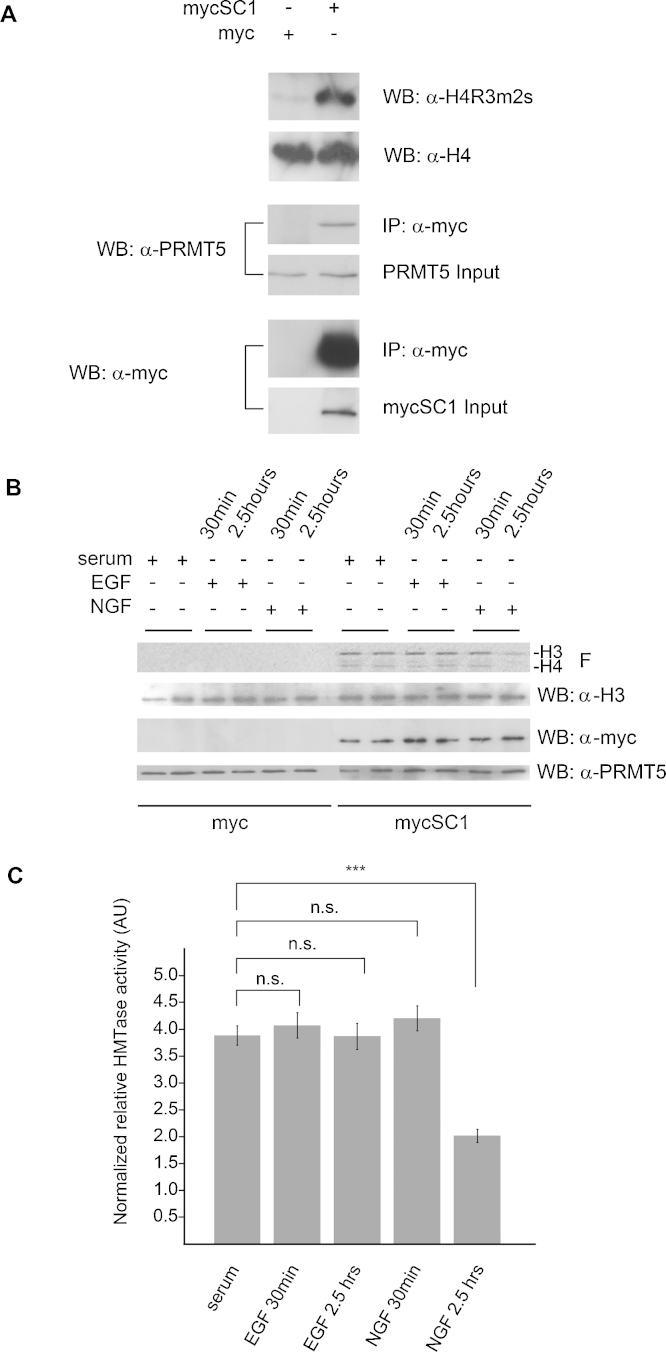
Fig. 1.
NGF down-regulates SC1/PRMT5-mediated HMTase activity in PC12 cells. (A) mycSC1 or Myc-tag alone were overexpressed in PC12 cells, the proteins IPed using anti-myc antibody and used for an in vitro HMTase assay with purified calf thymus histones. The products of HMTase reaction were probed with anti-H4R3me2s antibodies (top panel) and with anti-H4 antibodies to control for the histone loading (2nd panel from the top). Input, IPed and co-iped proteins were detected by using indicated antibodies to detect endogenous PRMT5 or mycSC1 (middle two and bottom two panels, respectively). (B) Transfected PC12 cells were cultured with serum, EGF or NGF as indicated. Overexpressed myc and mycSC1 were IPed and used for in vitro radioactive HMTase assay with purified calf thymus histones. Top panel: HMTase reaction products; 2nd, 3rd and bottom panels - Western blots probed with: anti-H3 antibody to control for the histone loading; anti-myc antibody to detect IPed mycSC1 protein, anti-PRMT5 antibody to detect PRMT5, respectively. (C) Quantification of normalized HMTase activity detected in PC12 cells grown in serum, EGF or NGF. Relative HMTase activity was normalized to PRMT5 levels in the lysates (means ± S.D., n = 3, t test, P < 0.001). AU: arbitrary units; n.s.: not significant; F: fluorogram; IP: immunoprecipitation; WB: Western blot.