Figure 3.
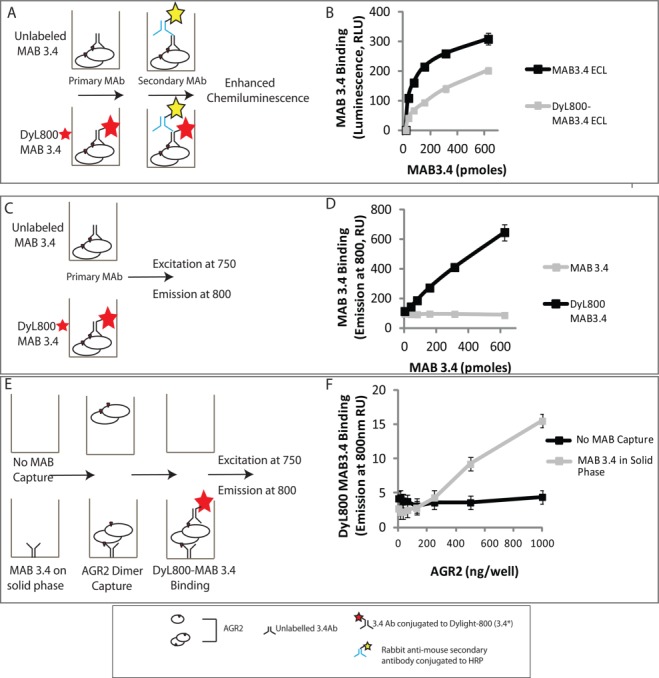
Developing a quantitative microtiter assay to measure AGR2 oligomerization. (A) Evaluation of the bioactivity of fluorescently labeled MAB3.4 in a luminescent-based ELISA. (B) MAB3.4 was left unconjugated or conjugated to DyL800 and after purification of the latter; the monoclonal antibodies were titrated into reactions containing AGR2 protein on the solid phase. The binding of MAB3.4 to AGR2 was measured using an anti-mouse IgG secondary antibody conjugated to peroxidase. The bioactivity of the monoclonal antibody (in relative luminescent units) is measured as a function of increasing MAB3.4 concentration. (C) Evaluation of the bioactivity of fluorescently labeled MAB3.4 in a fluorescence detection assay. (D) MAB3.4 was left unconjugated or conjugated to DyL800 and the monoclonal antibodies were titrated into reactions containing AGR2 protein on the solid phase. The bioactivity of the monoclonal antibody was measured as levels of emission at 800 nm a function of increasing MAB3.4 concentration. (E) Evaluation of the bioactivity of fluorescently labeled MAB3.4 to detect a potential AGR2 oligomer. (F) MAB3.4 was coated onto the solid phase and increasing amounts of oligomeric AGR2 were added to allow capture onto the solid phase. Fixed amounts of DyL800 MAB were added into reactions and the binding of DyL800-MAB3.4. The extent of oligomerization was quantified as levels of emission at 800 nm a function of increasing AGR2 protein concentration and is presented as an average from triplicate titrations. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
