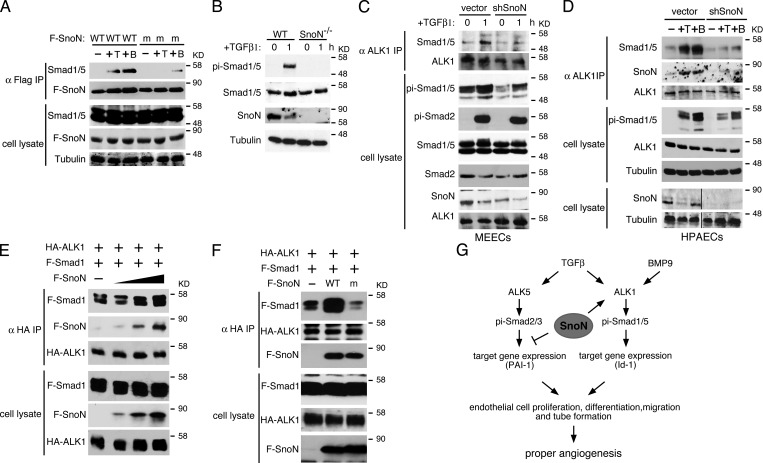
Figure 7.
SnoN facilitates the interaction of Smad1/5 with ALK1. (A) WT but not mutant SnoN associates with Smad1/5 in endothelial cells. Flag-tagged WT or mutant SnoN was transiently transfected into WT MEECs. 48 h after transfection, the cells were stimulated with or without either 100 pM TGF-β or 10 ng/ml BMP9 for 30 min. Interaction between Flag-SnoN (F-SnoN) and endogenous Smad1/5 was detected by Western blotting analysis of anti-Flag IP (top panels). The levels of these proteins in cell lysates are shown in the bottom panels. (B) SnoN−/− MEECs also display a reduced Smad1/5 phosphorylation. MEECs isolated from E11.5 WT or SnoN−/− embryos were treated with 100 pM TGF-β1 for 1 h, and phosphorylation of Smad1/5 detected by Western blotting. (C and D) Knocking down SnoN by shRNA reduces the binding of Smad1/5 to ALK1. WT MEECs (C) or WT HPAECs (D) infected with a retrovirus expressing shSnoN or vector control were treated with 100 pM TGF-β or 10 ng/ml BMP9 for 1 h and subjected to coimmunoprecipitation assay with anti-ALK1. Black lines in the bottom two cell lysate panels indicate the removal of intervening lanes for presentation purposes. (E) SnoN enhances the Smad1–5-ALK1 interaction. 293T cells were transfected with a fixed amount of HA-ALK1 and Flag-Smad1 (F-Smad1) and increasing amounts (0, 0.5, 1, 2 µg) of Flag-SnoN (F-SnoN). Smad1 and SnoN that associated with ALK1 were isolated by coimmunoprecipitation with anti-HA (ALK1) and detected by Western blotting with anti-Flag. (F) Mutant SnoN fails to enhance the Smad1–ALK1 interaction. HA-ALK1 and Flag-Smad1 were cotransfected with Flag-tagged WT SnoN or mSnoN into 293T cells. The Smad1–ALK1 interaction was measured as described in E. (G) Model of SnoN regulation of TGF-β and BMP9 signaling in endothelial cells. SnoN enhances activation of Smad1/5 by ALK1 while repressing the activity of the ALK5 pathway. By regulating the activity of the two pathways, SnoN maintains the proper level of endothelial cell proliferation, migration, and maturation.