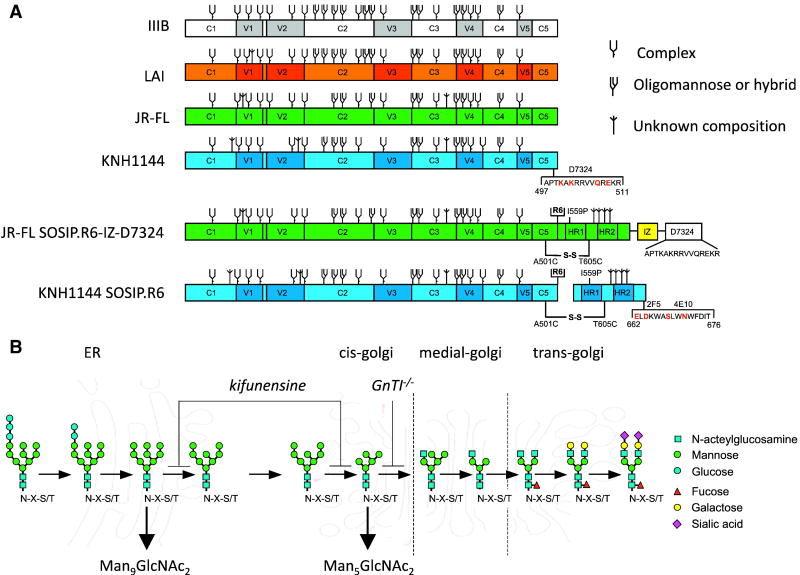
Figure 1.
Study design. (A) Schematic of the gp120 and gp140 proteins used in this study. Constructs based on the sequences from LAI, JR-FL and KNH1144 are indicated in orange, green and blue, respectively. KNH1144 gp120 was modified to reconstitute the epitope for D7324 in the C5 domain (substitutions: R500K, R502K and G507Q). JR-FL SOSIP.R6-IZ-D7324 gp140 and KNH1144 SOSIP.R6 MPER gp140 contain several modifications that have mostly been described elsewhere, including the A501C and T605C substitutions to create the SOS disulfide bond (Binley et al., 2000); the I559P substitution to promote trimerization (Sanders et al., 2002b); the GCN4-based isoleucine zipper (IZ) introduction to promote trimerization (Harbury, Kim, and Alber, 1994; Yang et al., 2000a; Yang et al., 2000b) followed by an D7324 epitope tag; the hexa-arginine (R6) cleavage site to enhance cleavage (Binley et al., 2002); and the MPER substitutions, A662E, G664D, N668S and T671N (indicated in red) to introduce the 2F5 and 4E10 epitopes into KNH1144 SOSIP.R6 gp140 (Dey et al., 2009). The N-linked glycan sites on gp120 and gp140 proteins produced in wild type mammalian cells are designated as oligomannose or complex, based on experimental determinations using IIIB gp120 (Leonard et al., 1990). It is assumed that the glycans present at analogous sites are processed similarly on the other gp120s, but we note that the N-glycan type (oligomannose vs complex) present at some sites depends on the study and isolate used (Cutalo, Deterding, and Tomer, 2004; Leonard et al., 1990; Zhu et al., 2000). Furthermore, some sites can be unoccupied in a only subset of molecules, or be occupied by both complex and oligomannose sugars (Cutalo, Deterding, and Tomer, 2004; Zhu et al., 2000). Sites that are present on LAI, JR-FL or KNH1144 gp120, but not on IIIB gp120, are designated as being of unknown glycan composition. The glycans on gp41 have not been characterized. (B) Schematic of the mammalian N-linked glycosylation pathway with the blocks in 293S GnTI-/- cells and in 293T cells treated with kifunensine indicated. The cellular sites at which processing occurs are also indicated.