Abstract
Aims
Sublingual buprenorphine and buprenorphine/naloxone are efficacious opioid dependence pharmacotherapies, but there are reports of their diversion and misuse by the intranasal route. The study objectives were to characterize and compare their intranasal pharmacodynamic and pharmacokinetic profiles.
Design
A randomized, double-blind, placebo-controlled, crossover study.
Setting
An in-patient research unit at the University of Kentucky.
Participants
Healthy adults (n=10) abusing, but not physically dependent on, intranasal opioids.
Measurements
Six sessions (72 hours apart) tested five intranasal doses [0/0, crushed buprenorphine (2, 8 mg), crushed buprenorphine/naloxone (2/0.5, 8/2 mg)] and one intravenous dose (0.8 mg buprenorphine/0.2 mg naloxone for bioavailability assessment). Plasma samples, physiological, subject- and observer-rated measures were collected before and for up to 72 hours after drug administration.
Findings
Both formulations produced time- and dose-dependent increases on subjective and physiological mu-opioid agonist effects (e.g. ‘liking’, miosis). Subjects reported higher subjective ratings and street values for 8 mg compared to 8/2 mg, but these differences were not statistically significant. No significant formulation differences in peak plasma buprenorphine concentration or time-course were observed. Buprenorphine bioavailability was 38–44% and Tmax was 35–40 minutes after all intranasal doses. Naloxone bioavailability was 24% and 30% following 2/0.5 and 8/2 mg, respectively.
Conclusions
It is difficult to determine if observed differences in abuse potential between intranasal buprenorphine and buprenorphine/naloxone are clinically relevant at the doses tested. Greater bioavailability and faster onset of pharmacodynamic effects compared to sublingual administration suggests a motivation for intranasal misuse in non-dependent opioid abusers. However, significant naloxone absorption from intranasal buprenorphine/naloxone administration may deter the likelihood of intranasal misuse of buprenorphine/naloxone, but not buprenorphine, in opioid-dependent individuals.
INTRODUCTION
Buprenorphine, a partial mu opioid agonist, is an effective treatment for opioid dependence [1, 2] when administered alone or combined with naloxone. Buprenorphine was first introduced for opioid dependence treatment in 1996 in France [3] and is registered for use in countries across Europe, North America, Asia and in Australia. Food and Drug Administration (FDA) approval of buprenorphine has greatly increased treatment access in the United States where prescriptions for buprenorphine (primarily buprenorphine/naloxone) increased from approximately 267 000 in 2004 to more than 3.3 million in 2008 [4]. Not surprisingly, as availability increased, so have reports of diversion and misuse [5].
Buprenorphine produces a dose-response that is characterized by a ceiling on the magnitude of its pharmacodynamic effects (e.g. respiratory depression; [6–9]) providing a more favorable safety profile than full agonists. However, human laboratory studies demonstrate that buprenorphine has abuse liability, as it can produce euphorigenic effects comparable to full opioid agonists and is self-administered by nondependent opioid users [10, 11]. Because of its lower intrinsic activity and high affinity, buprenorphine may precipitate opioid withdrawal in opioid-dependent individuals, thereby reducing its abuse liability [12–14]. This is supported by epidemiological data indicating that buprenorphine is infrequently (<3%) reported as the drug of choice among prescription opioid-dependent people seeking treatment [15]. The buprenorphine/naloxone combination product was developed to decrease further the abuse potential of buprenorphine and limit its parenteral (i.e., intravenous) diversion. Naloxone is virtually inactive sublingually [16] but, when injected, can precipitate withdrawal [17, 18]. In subjects without physical dependence, there are not clear differences in abuse liability between these formulations [10, 19, 20].
Diversion and misuse of both formulations have been reported. Specifically, buprenorphine and buprenorphine/naloxone tablets are being crushed and then taken by injection [21–23] or intranasally [24–26] in the United States and abroad. While studies have characterized the effects of buprenorphine sublingually and by injection [27–32], no studies, to date, have examined the profile of intranasal (i.e. snorting, inhalation) buprenorphine. The purpose of this study was to examine the intranasal pharmacodynamic and pharmacokinetic profile of crushed buprenorphine and buprenorphine/naloxone in intranasal opioid abusers without opioid physical dependence. The hypotheses were that intranasal buprenorphine/naloxone would have modestly decreased abuse potential compared to buprenorphine alone and that, like other opioids, both buprenorphine and naloxone would exhibit significant intranasal absorption.
METHODS
Subjects
Twelve recreational prescription opioid users were recruited by advertisements and admitted as in-patients. All were in good health according to medical history, physical examination, electrocardiogram and laboratory tests. Exclusion criteria included those with: seizure disorders, history of asthma or respiratory disorders, head injury, hypertension, cardiovascular disease, abnormal electrocardiogram or required daily prescribed medication. All subjects reported illicit opioid use (confirmed by urinalysis during multi-day intake) and intranasal as their preferred administration route. An opioid-negative urine sample was also required during screening in the absence of withdrawal symptoms to exclude opioid physical dependence. Individuals seeking treatment for substance abuse or successfully sustaining abstinence were excluded.
Two subjects were discharged before study completion for personal reasons or failure to comply with study procedures. Of the ten who completed (seven male, three female), all were Caucasian, with a mean [± standard error of the mean (SEM)] age of 31.2 ± 2.27 years. Subjects reported using illicit opioids 10 ± 2.3 days of the preceding 30 days. Average reported age of first use of illicit opioids was 16 ± 0.9 years with a lifetime history use of opioids of 7.7±1.7 years. Subjects also reported current use of cigarettes (n=9), alcohol (n=10), cocaine (n=6), sedatives/hypnotics/tranquilizers (n=6), marijuana (n=8) and amphetamines (n=1). The University of Kentucky (UK) Institutional Review Board approved this study; all subjects gave written informed consent and were paid for participation. This study was conducted in accordance with the Helsinki guidelines for ethical human research. A Certificate of Confidentiality was obtained from the National Institutes of Health.
Drugs
This study was performed under an investigator-initiated Investigational New Drug Application (#69214) with the FDA. All study medications were stored and prepared in the UK Investigational Pharmacy. Subutex® (2 and 8 mg tablets and matched placebos; all white in color) was obtained through the National Institute on Drug Abuse. Suboxone® (2/0.5 and 8/2 mg tablets and matched placebos; all white in color) was imported from Hull, England (Reckitt Benckiser Pharmaceuticals) because in the United States Suboxone® is orange, which would have broken the subject blind. These doses were selected for testing because they are the currently marketed dose strengths and are available for clinical use and misuse. Individual ampoules containing buprenorphine/naloxone solution (4 mg buprenorphine/1 mg naloxone/1 ml) were obtained through the NIDA drug supply (Murty Pharmaceuticals, Lexington, KY, USA) and diluted 1:5 for a final dose of 0.8 mg buprenorphine/0.2 mg naloxone/1 ml for intravenous administration. Intravenous doses of both drugs were included primarily to assess bioavailability. The intravenous naloxone dose was selected because 0.2 mg naloxone is sufficient to precipitate withdrawal in opioid dependent individuals; thus, the expected plasma concentrations would be clinically informative and relevant. The intravenous buprenorphine dose was selected to maintain the 4:1 ratio of buprenorphine and naloxone used in the marketed medication.
Study Design
This 3.5 week in-patient study employed a randomized, double-blind, within-subject, placebo-controlled design. It was conducted at the Clinical Research Development and Operations Center (CR DOC), a research unit in the UK hospital. Subjects participated in six 6.5-hour experimental sessions scheduled minimally 72 hours apart.
Experimental Sessions
Following admission, subjects were familiarized with and trained on all procedures. Subjects were maintained on a caffeine-free diet, allowed a light breakfast 2 hours before session, and could smoke up to 30 minutes before session. Females were tested weekly for pregnancy with no positive results. On session days, subjects received powder from crushed placebo, buprenorphine (2 or 8 mg) or buprenorphine/naloxone (2/0.5 or 8/2 mg) tablets of equivalent volume (100 mg). The placebo dose contained powder from the matched placebo tablets of both buprenorphine (50%) and buprenorphine/naloxone (50%). Subjects transferred the powder to a mirror, split the powder into two lines, and snorted one line through each nostril using a straw. Subjects completed computerized questionnaires using a keyboard and/or mouse. A trained research assistant used a keyboard to initiate tasks and to enter observer-rated measures. Baseline data were collected for 30 minutes prior and 6 hours after drug administration (at 0900). Table 1 details the timing of all pharmacodynamic measures.
Table 1.
Study task timeline.
| Dependent Measure |
Time (relative to drug administration) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session | Post-Session | ||||||||||||||||||||||||
| Minutes | Hours | ||||||||||||||||||||||||
| −25 | 5 | 10 | 15 | 20 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | 330 | 360 | 8 | 12 | 24 | 48 | 72 | |
| Visual Analog Scale | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Subject-rated Adjectives | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| ARCI | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| Drug Identification | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Street Value | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Observer-rated Adjectives | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Maddox Wing | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| DSST | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Snorting Experience | X | ||||||||||||||||||||||||
| Pupil Diameter | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Respiration Rate | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
ARCI: Addiction Research Center Inventory; DSST: digit symbol substitution task
Subject and Observer-Rated Measures
Subject-rated measures included: six visual analog scales (VAS) rated from 0 (‘not at all’) – 100 (‘extremely’; [33]); the Addiction Research Center Inventory (ARCI) short form [34]; street value questionnaire; a 25-item adjective checklist that encompassed both an Agonist scale and mixed Agonist-Antagonist scale [16, 35]; and an observer-rated opioid adjective rating scale.
Subjects also completed two locally developed questionnaires to characterize the sensations related to nasal inhalation of the test drugs using a 5-point Likert scale: (i) ‘When I snorted this drug it tasted or smelled…’ sweet, salty, sour, bitter, like metal, like medicine, like chalk, like fruit, bad, good; and (ii) ‘When I snorted this drug, my nose or throat felt…’ burning, tingling, itching, pain, congestion, numbness, stinging, thirsty, dry mouth. This second questionnaire also included a ‘yes’ or ‘no’ question ‘Was it difficult to snort the amount of powder provided?’
Performance and Ocular Tasks
The digit symbol substitution task (DSST) was used to measure information processing [36]. The Maddox-Wing test (Model CE0120, Clement Clarke Ltd., London, UK) was used to assess ocular exophoria or under convergence [33].
Physiological Measures
Oxygen saturation, heart rate and blood pressure were collected every min using a Dinamap Non-Invasive Patient Monitor (GE Medical Systems, Tampa, FL) for 30 minutes before and for 6 hours after drug administration. Respiratory rate was determined by counting the number of breaths within 30 seconds and multiplying by 2. Pupil diameter was determined using a pupillometer (NeurOptics, San Clemente, CA) in constant lighting conditions.
Blood Sample Collection and Pharmacokinetic Analysis
Intravenous catheter(s) were placed into the antecubital vein(s) prior to the start of session, one for intranasal sessions and two (in separate arms) for the intravenous session (the second for drug administration). Catheters remained in place for up to 72 hours and were flushed regularly to maintain patency. Blood samples (7 ml/sample) were collected into two 4-ml green heparinized vacutainers for determination of buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, norbuprenorphine-3-glucuronide and naloxone levels. Samples were collected at baseline and 5, 10, 15, 20, 30, 45 minutes, 1, 2, 4, 8, 12, 24, 48 and 72 hours post-drug administration and one sample at 2 minutes during the intravenous session. Vacutainers were inverted 8–10 times and centrifuged (3000 g × 15 minutes) immediately to prevent hemolysis. Plasma was transferred to a vial, stored at −80°C, shipped to the University of Utah Center for Human Toxicology and assayed for buprenorphine (and metabolites) and naloxone (the latter only for those sessions testing buprenorphine/naloxone). Analysis was performed as previously described [37]. This technique reliably quantifies buprenorphine (and metabolites) and naloxone levels with a lower limit of quantitation (LLOQ) of 0.1 ng/ml and 0.025 ng/ml, respectively.
Statistical Analysis
All measures were analyzed as raw time-course data with two-factor within-subject analysis of variance [ANOVA; dose (five levels) × time (intervals in Table 1)] followed by Tukey post-hoc analyses. Physiological measures collected every minute were first averaged across time to yield intervals (5–30 minutes) corresponding to collection of subjective reports. Peak scores (minimum or maximum depending upon the a priori predicted direction of effect) were derived from time-course data and analyzed using one-factor ANOVA for dose. Planned comparisons with Bonferroni corrections were used for active dose comparisons to placebo and between formulations.
Buprenorphine concentrations were analyzed using 3-factor ANOVA [dose (two levels) by formulation (two levels) by time]. Naloxone concentrations were analyzed for 8 hours after buprenorphine/naloxone with two-factor ANOVA [dose (two levels) × time]. Plasma area-under-the-curve (AUC) was calculated by the trapezoidal rule (0–72 hours). Mean maximum concentrations (Cmax) and time-to-maximum concentrations (Tmax) were calculated for buprenorphine, its metabolites, and naloxone. Observed absolute bioavailability (Fobs) of intranasal buprenorphine and naloxone was determined as follows: Fobs = (AUC intranasal/AUC intravenous) × (dose intravenous/dose intranasal). Values below the LLOQ were scored as zero for mean concentrations and AUC calculations. The elimination rate constant (λz) was estimated by linear regression from a natural log linear plot of data from 12, 24 and 48 hours for 8 and 8/2 mg buprenorphine and data from 45 minutes, 1, 2 and 4 hours naloxone (the terminal post-distribution phases). Buprenorphine concentrations below the LLOQ at these times or with a slope approaching 0 for these time points were excluded from the λz calculation. For low doses of buprenorphine and all buprenorphine metabolites, λz was not calculated due to insufficient concentrations at these times. The terminal half-life (t1/2) was calculated as 0.693/λz. One-factor ANOVA along with Tukey tests were used to determine differences among the buprenorphine parent and metabolite conditions on pharmacokinetic parameters. Paired t-tests were used to compare pharmacokinetic parameters for naloxone. All ANOVA models were run with SAS 9.1 Proc Mixed software for Windows and were considered significant when P < 0.05.
RESULTS
Physiological Measures
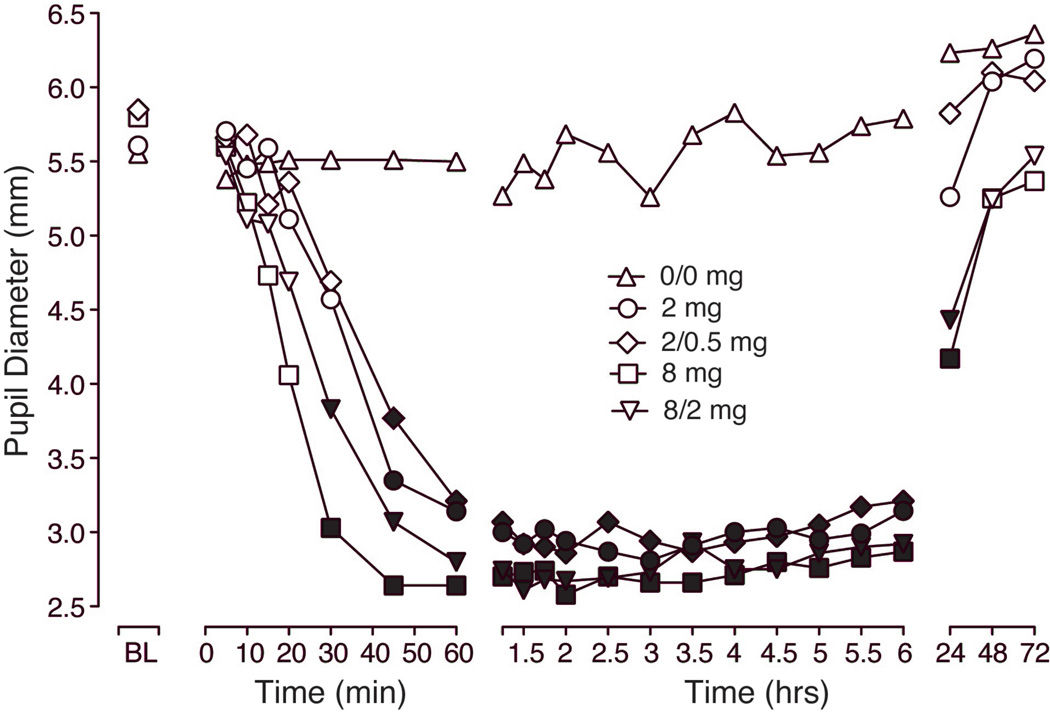
Figure 1 illustrates the time-course for pupil diameter over the first 72 hours after intranasal dosing. There were significant effects of dose and time (see figure legend for statistical outcomes). Significant miosis was observed within 30 minutes of dosing after 8 and 8/2 mg, but not until 45 minutes for the lower doses (2 and 2/0.5 mg; Tukey test P < 0.05). Data from the first hour reveal a slightly earlier onset for buprenorphine alone compared to the same doses of the combination; however, these were not significant. Tukey post-hoc analyses revealed that miosis lasted for up to 6 hours for the low doses (2 and 2/0.5 mg), but was still evident 24 hours after administration of 8 and 8/2 mg. Similarly, peak analysis of minimum diameter scores revealed a main effect of dose (Table 2) with all active doses significantly different from placebo (P < 0.0001; planned comparisons).
Figure 1.
Data shown are mean (n=10) values for pupil diameter (mm) at baseline and for up to 72 hours after intranasal administration of placebo (0/0 mg), buprenorphine (2 and 8 mg) and buprenorphine/naloxone (2/0.5 and 8/2 mg). Significant main effects were found for both dose (F(4,36) = 32.7; P < 0.0001) and time (F(22,195) = 36.0; P < 0.0001). Filled symbols indicate significant differences from placebo based on Tukey tests (P < 0.05).
Table 2.
Peak scores for physiological, subjective and objective measures (n=10).
| Buprenorphine (mg) | Buprenorphine/Naloxone (mg) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 8 | 2/0.5 | 8/2 | ||
| Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | ||
| Physiological | ||||||
| Pupil Diameter* | 4.65 (0.38) | 2.64 (0.08) | 2.38 (0.10) | 2.61 (0.12) | 2.39 (0.12) | |
| Oxygen Sat (%)* | 97.1 (0.68) | 95.7 (0.30) | 94.6 (0.47) | 95.8 (0.35) | 95.5 (0.43) | |
| Respiratory Rate* | 13.2 (0.95) | 11.8 (0.47) | 11 (0.45) | 11.2 (0.61) | 10.8 (0.44) | |
| Systolic BP | 102 (2.6) | 107 (2.8) | 106 (3.7) | 105 (3.9) | 105 (2.7) | |
| Diastolic BP | 57 (1.7) | 59 (1.3) | 58 (2.1) | 58 (2.2) | 58 (1.9) | |
| Subjective | ||||||
| VAS | Like* | 2.7 (1.48) | 27 (7.37) | 46.3 (6.21) | 25 (6.10) | 43.6 (6.10) |
| Drug Effect* | 4.1 (1.86) | 26.6 (6.38) | 40.9 (5.00) | 30.5 (5.62) | 36.3 (5.97) | |
| High* | 3.3 (1.51) | 24.7 (7.36) | 39.3 (6.04) | 23.1 (6.38) | 35.5 (6.27) | |
| Good* | 3.3 (1.54) | 25 (7.24) | 39.8 (6.18) | 24.5 (6.13) | 35.5 (6.17) | |
| Bad* | 0.6 (0.50) | 4.4 (2.65) | 11.2 (4.45) | 9.6 (3.32) | 8.4 (2.83) | |
| Street Value* | 1.28 (0.68) | 13.9 (3.60) | 22.5 (3.83) | 12.0 (3.55) | 18.8 (3.91) | |
| Adjectives | Agonist* | 10.2 (1.40) | 14.3 (1.58) | 16.9 (1.52) | 14.7 (2.19) | 17.2 (2.24) |
| Tingling* | 0 (0) | 0.3 (0.21) | 0.6 (0.22) | 0.7 (0.21) | 0.3 (0.15) | |
| Itchy* | 0 (0) | 1.5 (0.48) | 1.9 (0.41) | 1.6 (0.4) | 1.8 (0.39) | |
| Nodding* | 0.3 (0.3) | 0.8 (0.47) | 2.1 (0.41) | 1.2 (0.39) | 1.4 (0.48) | |
| Relaxed* | 2.1 (0.18) | 2.2 (0.20) | 2.8 (0.25) | 2.3 (0.21) | 2.7 (0.21) | |
| Coasting* | 0.3 (0.3) | 0.9 (0.35) | 1.1 (0.31) | 1 (0.30) | 0.9 (0.41) | |
| Talkative* | 1.5 (0.22) | 2.2 (0.36) | 2.5 (0.24) | 1.5 (0.37) | 2.2 (0.3) | |
| Dry Mouth* | 0.3 (0.21) | 1.2 (0.33) | 1.4 (0.34) | 1.2 (0.25) | 1.3 (0.40) | |
| Good Mood* | 2.1 (0.18) | 2.8 (0.2) | 3.1 (0.23) | 2.5 (0.22) | 2.9 (0.23) | |
| Energetic* | 1.1 (0.31) | 1.9 (0.35) | 2.3 (0.29) | 1.8 (0.36) | 1.9 (0.33) | |
| Floating* | 0 (0) | 0.3 (0.21) | 0.5 (0.22) | 0.7 (0.26) | 0.8 (0.33) | |
| Pleasant | 0 (0) | 0.2 (0.13) | 0.1 (0.1) | 0.2 (0.13) | 0.7 (0.40) | |
| Heavy | 0.2 (0.2) | 0.9 (0.35) | 1 (0.39) | 1.2 (0.39) | 0.9 (0.38) | |
| Drive | 1.2 (0.36) | 1.4 (0.22) | 1.8 (0.36) | 1.5 (0.22) | 1.6 (0.31) | |
| Sweating | 0 (0) | 0.1 (0.1) | 0.3 (0.21) | 0.7 (0.42 | 0.1 (0.1) | |
| Carefree | 1.1 (0.4) | 1.4 (0.4) | 1.7 (0.47) | 1.5 (0.4) | 1.6 (0.5) | |
| Lightheaded | 0.4 (0.31) | 0.7 (0.26) | 0.7 (0.37) | 1.2 (0.36) | 1.1 (0.38) | |
| ARCI | PCAG* | 5.1 (0.72) | 5.1 (0.77) | 6.3 (0.78) | 7.1 (1.06) | 6 (0.95) |
| AMPH* | 3.2 (0.57) | 4.8 (0.76) | 4.8 (0.70) | 4.1 (0.77) | 4.6 (0.81) | |
| MBG* | 3.4 (0.76) | 8.2 (1.72) | 8.4 (1.54) | 6 (1.38) | 6.6 (1.18) | |
| LSD* | 3.6 (0.16) | 5.2 (0.59) | 5.2 (0.44) | 5 (0.56) | 5.2 (0.33) | |
| Sedation | 2 (0.77) | 2.2 (0.73) | 3.2 (0.95) | 3.8 (1.1) | 2.6 (0.92) | |
| Nose/Throat | Itching | 0 (0) | 0.2 (0.13) | 0.9 (0.48) | 0.3 (0.21) | 0.4 (0.22) |
| Numbness | 0 (0) | 0 (0) | 0.2 (0.13) | 0 (0) | 0 (0) | |
| Stinging | 0.8 (0.33) | 0.6 (0.16) | 0.9 (0.28) | 1.5 (0.37) | 1 (0.30) | |
| Taste /Smell | Sweet* | 1.4 (0.4) | 0.4 (0.16) | 0.3 (0.15) | 1.6 (0.37) | 1 (0.21) |
| Bitter* | 0.3 (0.15) | 0.7 (0.15) | 1.3 (0.37) | 0.6 (0.34) | 0.6 (0.22) | |
| Like Medicine* | 0.4 (0.16) | 1.1 (0.31) | 1.5 (0.31) | 0.3 (0.15) | 0.5 (0.22) | |
| Like Chalk* | 0.1 (0.1) | 1 (0.33) | 0.8 (0.25) | 0.3 (0.15) | 0.2 (0.13) | |
| Like Fruit* | 2.2 (0.44) | 0.4 (0.22) | 0.2 (0.13) | 2.3 (0.42) | 2.3 (0.45) | |
| Observer Rated | ||||||
| Observer Adjectives | Agonist* | 6.6 (0.67) | 10.6 (0.82) | 10.8 (0.81) | 10.4 (1.36) | 10.9 (0.94) |
| Itchy* | 0 (0) | 0.9 (0.28) | 1.5 (0.27) | 0.9 (0.28) | 1.2 (0.25) | |
| Nodding* | 0.1 (0.1) | 0.4 (0.31) | 1 (0.26) | 0.9 (0.35) | 1.3 (0.37) | |
| Coasting* | 0.1 (0.1) | 0 (0) | 0.3 (0.21) | 0.6 (0.31) | 0.2 (0.2) | |
| Talkative* | 1.3 (0.15) | 2.3 (0.26) | 2.1 (0.18) | 1.8 (0.2) | 1.8 (0.2) | |
| Energetic* | 0.1 (0.1) | 1 (0.21) | 1.1 (0.1) | 0.8 (0.25) | 0.5 (0.22) | |
| Good Mood | 1.4 (0.16) | 2 (0.15) | 2 (0.21) | 1.8 (0.13) | 1.9 (0.18 | |
| Sleepy | 1.1 (0.31) | 0.8 (0.29) | 1.5 (0.31) | 1.7 (0.37) | 1.7 (0.26) | |
| Performance | ||||||
| Maddox Wing* | 5.1 (1.23) | 12.2 (1.91) | 12.8 (2.23) | 12.8 (1.98) | 12.7 (1.74) | |
| DSST | Attempted* | 33.2 (1.15) | 31 (1.43) | 31.4 (1.29) | 30.5 (1.44) | 31.3 (0.75) |
| Correct* | 32 (1.23) | 29 (1.41) | 27.6 (1.4) | 28.9 (1.64) | 30 (0.98) | |
Asterisk (*) indicates a significant main effect of dose (P < 0.05).
Bold numbers indicate significant differences from placebo (P < 0.05).
Boxed numbers indicate a significant difference between corresponding doses of the two formulations (i.e., 2 vs. 2/0.5 and 8 vs. 8/2; P < 0.05).
Peak maximum scores are reported for all measures except pupil diameter, oxygen saturation, respiratory rate and digit symbol substitution task (DSST) performance for which the nadir is reported.
AMPH: amphetamines, MBG: morphine-benzedrine group; LSD: lysergic acid diethylamide; PCAG: pentobarbital-chlorpromazine-alcohol general; ARCI: Addiction Research Center Inventory; VAS: visual analog scale; SEM: standard error of the mean.
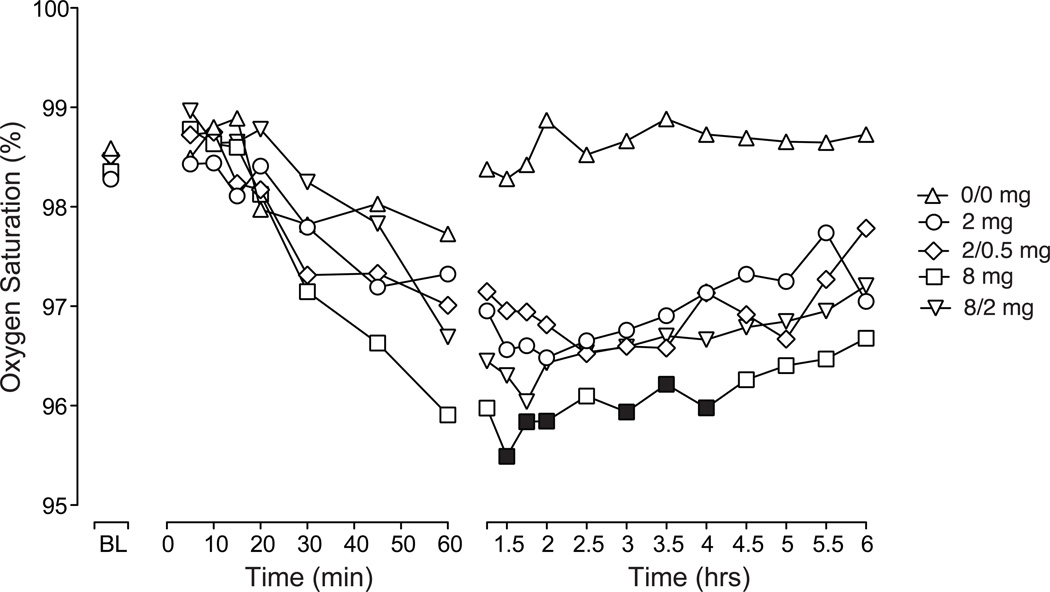
Figure 2 illustrates the time-course for oxygen saturation over the first 6 hours after intranasal dosing. There were significant effects of dose and time (see figure legend for statistics). While all active doses decreased oxygen saturation compared to placebo, Tukey post-hoc analyses revealed significant differences for only the 8 mg dose that occurred after peak effect was reached (1.5 – 4 hours). Peak analyses revealed significant dose effects and planned comparisons revealed a reduction in oxygen saturation for all active doses compared to placebo (P < 0.05; Table 2). Similarly, there was a significant effect of dose on respiratory rate for both time-course and peak analysis (see Table 2) with the 8, 2/0.5 and 8/2 mg doses significantly decreased compared to placebo (P < 0.05; planned comparisons). Time-course analyses revealed no significant dose effects for heart rate, systolic or diastolic blood pressure. However, planned comparisons revealed that the peak increase in systolic and diastolic blood pressure following 2 mg buprenorphine was significantly greater than placebo (P < 0.05).
Figure 2.
Data shown are mean (n=10) values for oxygen saturation at baseline and for up to 6 hours after intranasal administration of placebo (0/0 mg), buprenorphine (2 and 8 mg) and buprenorphine/naloxone (2/0.5 or 8/2 mg). Significant main effects were found for dose (F(4,36) = 8.65; P < 0.0001) and time (F(20,180) = 5.65; P < 0.0001). Filled symbols indicate significant differences from placebo based on Tukey tests (P < 0.05).
Subject-Rated Measures
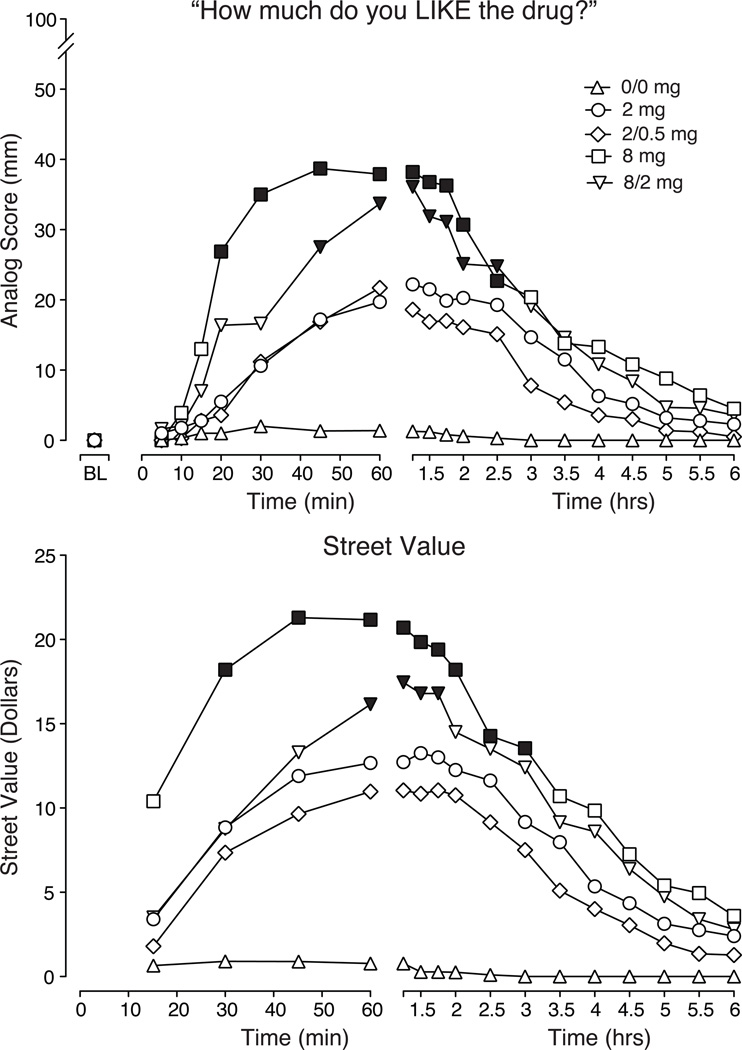
Time-course data for the first 6 hours after intranasal dosing for the visual analog rating ‘How much do you LIKE the drug?’ are illustrated in Fig. 3 (top panel). Analyses of the time course data for ratings of ‘like’, ‘high’, ‘drug effect’ and ‘good’ (data not shown) revealed significant dose dependent effects (P < 0.001). Compared to placebo, a significant increase in ratings of “liking” was observed by 20 minutes after the 8 mg dose and 45 minutes after the 8/2 mg dose (Tukey test P < 0.05), while 2 and 2/0.5 mg doses were not significantly different from placebo. For both 8 and 8/2 mg, this effect persisted for 2.5 hours post-dosing. Peak analysis of VAS measures revealed significant dose effects for ‘high’, ‘drug effect’, ‘like’, ‘good’ and ‘bad’ (P < 0.01; results from planned comparison described in Table 2).
Figure 3.
Data shown are mean scores (n=10) for visual analog ratings of ‘How much do you LIKE the drug?’ (top panel) and street value (US$; bottom panel). For ratings of ‘liking’, significant main effects were found for dose (F(4,36) = 9.18; P < 0.0001) and time (F(24,216) = 9.89; P < 0.0001). For street value ratings, significant main effects were found for dose (F(4,36) = 10.89; P < 0.0001) and time (F(15,135) = 15.86; P < 0.0001). Filled symbols indicate significant differences from placebo based on Tukey tests (P < 0.05).
Figure 3 (bottom panel) illustrates the time-course for street value estimates for the intranasal test conditions. There was a significant dose dependent increase on ratings of street value for both the time course and peak analyses (see Table 2). The 8 mg dose was rated, on average, as having the highest value during the period of drug onset. Peak analyses revealed that all active doses differed from placebo (Table 2; P < 0.05; planned comparisons).
Time-course analyses revealed significant dose effects for a number of prototypic opioid agonist symptoms, including tingling, itchy, pleasant, talkative, dry mouth, good mood, energetic and sweating, and on the composite Agonist scale (P < 0.05). No significant effects were found for the mixed agonist-antagonist scale. Similarly, peak score analyses revealed endorsement of typical mu agonist effects as a function of dose, including tingling, itchy, nodding, relaxed, coasting, talkative, dry mouth, good mood, energetic and floating and are reflected in the composite Agonist scale (P < 0.05); planned comparisons are shown in Table 2. Both the ARCI Benzedrine and morphine-benzedrine group (MBG) scales increased significantly as a function of dose (P < 0.05) on the time course analysis. Peak score analyses revealed significant increases for the pentobarbital-chlorpromazine-alcohol-general (PCAG), amphetamine, MBG and lysergic acid diethylamide (LSD) scales compared to placebo (P < 0.05; see Table 2).
Analysis of the results from the questionnaire on sensations in the nose and throat after drug administration revealed no significant main effect of dose. However, planned comparisons revealed that 8 mg buprenorphine increased ratings of ‘itching’ and ‘numbness’ compared to placebo, and 2/0.5 mg buprenorphine/naloxone produced more ‘stinging’ than placebo. Results from the questionnaire querying subjects about the taste of the drug revealed significant main effects of dose for ‘sweet’, ‘bitter’, ‘like medicine’, ‘like chalk’ and ‘like fruit’ (P < 0.05; Table 2). Buprenorphine 8 mg produced more ‘numbness’ than 8 mg buprenorphine/naloxone, 2/0.5 mg buprenorphine/naloxone produced more ‘stinging’ than 2 mg buprenorphine. Both doses of buprenorphine/naloxone tasted more ‘like fruit’ and ‘sweet’ than the corresponding buprenorphine doses, and both doses of buprenorphine alone tasted more ‘like chalk’ and ‘like medicine’ than the corresponding buprenorphine/naloxone doses. The high dose of buprenorphine alone was also more ‘bitter’ than the high dose of buprenorphine/naloxone. Four subjects reported that it was difficult to snort the amount of powder during at least one experimental session (on 13 of 50 sessions).
Observer-Rated Measures
Time-course analyses of the observer-rated scales revealed dose-related increases on itchy, talkative and energetic (P < 0.05). Peak analysis revealed significant increases of observer ratings of itchy, nodding, coasting, talkative and energetic (P < 0.05; Table 2) also reflected by the composite Agonist Scale (P < 0.01; see Table 2).
Ocular and Performance Measures
There was a significant effect of dose on Maddox Wing scores for both the time-course and peak analysis (see Table 2). Maddox Wing scores for all active doses were significantly higher than placebo (P < 0.01; planned comparisons).
Analysis of the DSST time-course data revealed a main effect of dose for number of trials attempted and number of correct trials (P < 0.05). Peak DSST scores decreased as a function of dose for the number of trials attempted and the number of trials correct (P < 0.05; Table 2).
Pharmacokinetic Outcomes
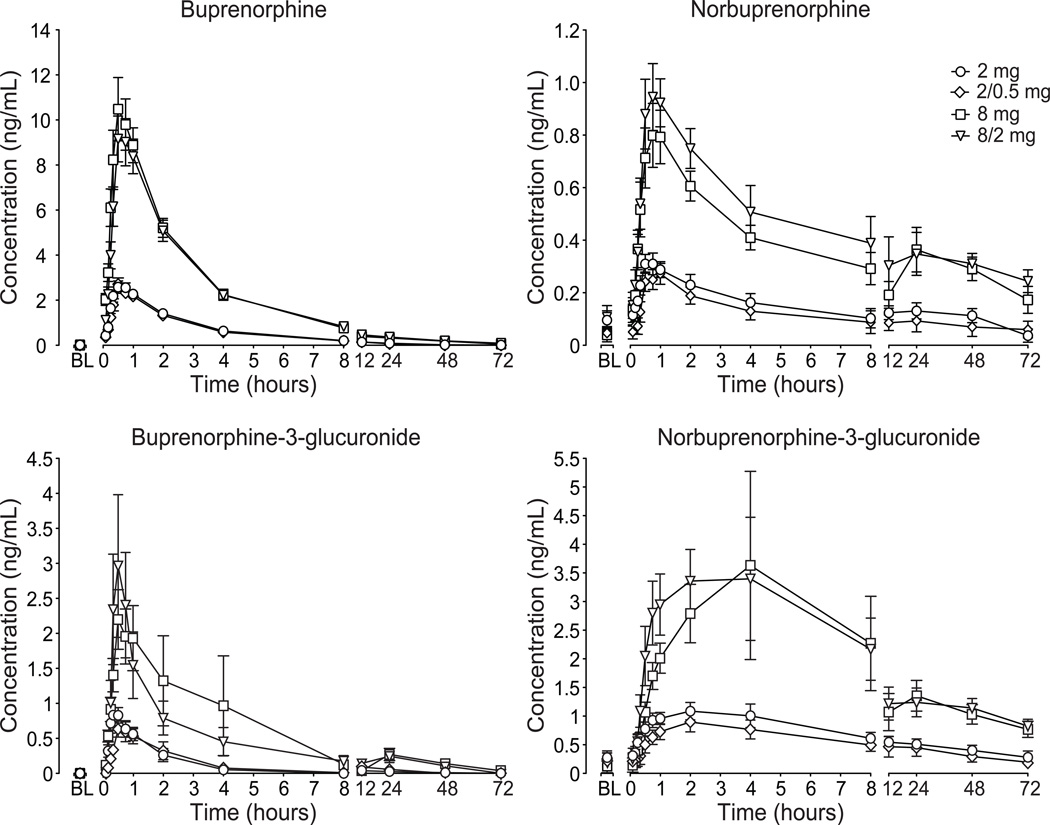
Figure 4 illustrates the 72-hour time-course for buprenorphine (top left), norbuprenorphine (top right), buprenorphine-3-glucuronide (bottom left) and norbuprenorphine-3-glucuronide (bottom right) as a function of dose; key outcome measures are shown in Table 3. Analysis of the plasma buprenorphine and the metabolites time course, AUC and Cmax revealed significant dose effects (P < 0.0001) with 8 and 8/2 mg greater than 2 and 2/0.5 mg, respectively. No differences for Tmax, t1/2 or bioavailability were observed for buprenorphine among the four active conditions. Buprenorphine concentrations returned to baseline levels (<LLOQ) prior to the start of the subsequent session (with the exception of three sessions in which buprenorphine concentration was slightly above the LLOQ).
Figure 4.
Data illustrate mean (n=10) values ± standard area of the mean for plasma concentrations of buprenorphine (top left), norbuprenorphine (top right), buprenorphine-3-glucuronide (bottom left) and norbuprenorphine-3-glucuronide (bottom right). Plasma concentrations of buprenorphine and metabolites were analyzed for up to 72 hours after drug administration.
Table 3.
Pharmacokinetic parameters of buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, norbuprenoprhine-3-glucuronide and naloxone after intranasal buprenorphine and buprenorphine/naloxone.
| Buprenorphine | Buprenorphine/Naloxone | |||
|---|---|---|---|---|
| 2 mg | 8 mg | 2/0.5 mg | 8/2 mg | |
| Tmax (min) | ||||
| Buprenorphine | ||||
| Mean (SEM) | 38 (4.36) | 34.5 (3.91) | 37.5 (4.03) | 39 (4.00) |
| Range | 20–60 | 15–60 | 30–60 | 30–60 |
| Norbuprenorphine | ||||
| Mean (SEM) | 41.5 (9.34) | 183 (140) | 474 (427) | 75 (20.0) |
| Range | 10–120 | 30–1440 | 30–4320 | 30–240 |
| Buprenorphine-3-glucuronide | ||||
| Mean (SEM) | 34.5 (9.67) | 52.5 (20.9) | 46.5 (8.79) | 58 (20.7) |
| Range | 15–120 | 30–240 | 30–120 | 20–240 |
| Norbuprenorphine-3-glucuronide | ||||
| Mean (SEM) | 99 (18.9) | 442 (274) | 161 (62.9) | 126 (43.9) |
| Range | 20–240 | 30–2880 | 30–720 | 20–480 |
| Naloxone | ||||
| Mean (SEM) | - | - | 18 (1.9) | 20 (2.6) |
| Range | - | - | 10–30 | 5–30 |
| Cmax (ng/mL) | ||||
| Buprenorphine | ||||
| Mean (SEM) | 2.82 (0.2) | 11.2 (1.20) | 2.79 (0.25) | 9.86 (0.93) |
| Range | 2.02–3.82 | 4.94–16.31 | 1.67–4 | 5.49–14.09 |
| Norbuprenorphine | ||||
| Mean (SEM) | 0.35 (0.04) | 0.92 (0.11) | 0.3 (0.04) | 1.1 (0.12) |
| Range | 0.18–0.54 | 0.5–1.41 | 0.13–0.47 | 0.48–1.76 |
| Buprenorphine-3-glucuronide | ||||
| Mean (SEM) | 1.03 (0.15) | 2.87 (0.65) | 0.82 (0.15) | 3.76 (1.0) |
| Range | 0.54–1.94 | 0.56–7.35 | 0.21–1.71 | 0.46–8.87 |
| Norbuprenorphine-3-glucuronide | ||||
| Mean (SEM) | 1.19 (0.18) | 4.04 (1.60) | 0.94 (0.17) | 4.4 (1.03) |
| Range | 0.49–2.52 | 1.14–18.2 | 0.22–1.93 | 0.68–12.4 |
| Naloxone | ||||
| Mean (SEM) | - | - | 0.39 (0.03) | 1.60 (0.14) |
| Range | - | - | 0.26–0.52 | 1.0–2.6 |
| AUC0–72 hrs | ||||
| Buprenorphine | ||||
| Mean (SEM) | 11.3 (1.19) | 43.6 (3.26) | 9.83 (1.12) | 44.4 (3.31) |
| Range | 8.52–20.6 | 26.5–64.6 | 4.01–16.0 | 31.1–62.1 |
| Norbuprenorphine | ||||
| Mean (SEM) | 8.14 (1.87) | 21.4 (3.64) | 6.10 (2.40) | 24.4 (4.22) |
| Range | 0.18–17.3 | 10.1–48.4 | 0.11–18.7 | 11.7–57.3 |
| Buprenorphine-3-glucuronide | ||||
| Mean (SEM) | 2.48 (0.77) | 16.8 (4.04) | 3.18 (1.02) | 13.9 (4.25) |
| Range | 0.39–6.89 | 0.99–37.3 | 0.39–8.14 | 0.79–45.0 |
| Norbuprenorphine-3-glucuronide | ||||
| Mean (SEM) | 34.9 (6.44) | 93.1 (18.1) | 27.7 (8.39) | 96.5 (15.7) |
| Range | 9.25–79.1 | 30.6–233 | 6.48–97.9 | 35.1–214 |
| Naloxone | ||||
| Mean (SEM) | - | - | 0.40 (0.06) | 2.02 (0.29) |
| Range | - | - | 0.24–0.94 | 1.34–4.52 |
| t1/2 | ||||
| Buprenorphine (hrs) | ||||
| Mean (SEM) | - | 29.47 (2.18) | - | 30.84 (2.61) |
| Range | - | 20.5–38.5 | - | 22.2–41.7 |
| Norbuprenorphine (hrs) | ||||
| Mean (SEM) | - | - | - | - |
| Range | - | - | - | - |
| Buprenorphine-3-glucuronide | ||||
| Mean (SEM) | - | - | - | - |
| Range | - | - | - | - |
| Norbuprenorphine-3-glucuronide | ||||
| Mean (SEM) | - | - | - | - |
| Range | - | - | - | - |
| Naloxone (min) | ||||
| Mean (SEM) | - | - | 43.4 (3.87) | 55.8 (2.64) |
| Range | - | - | 31–67 | 41–70 |
| Bioavailability (%) | ||||
| Buprenorphine | ||||
| Mean (SEM) | 44 (5.5) | 41 (3.7) | 38 (5.9) | 41 (3.7) |
| Range | 17–63 | 20–61 | 15–77 | 26–77 |
| Norbuprenorphine | ||||
| Mean (SEM) | - | - | - | - |
| Range | - | - | - | - |
| Buprenorphine-3-glucuronide | ||||
| Mean (SEM) | - | - | - | - |
| Range | - | - | - | - |
| Norbuprenorphine-3-glucuronide | ||||
| Mean (SEM) | - | - | - | - |
| Range | - | - | - | - |
| Naloxone | ||||
| Mean (SEM) | - | - | 24 (3.0) | 30 (2.9) |
| Range | - | - | 14–41 | 20–49 |
(-) indicates data not available; SEM: standard error of the mean.
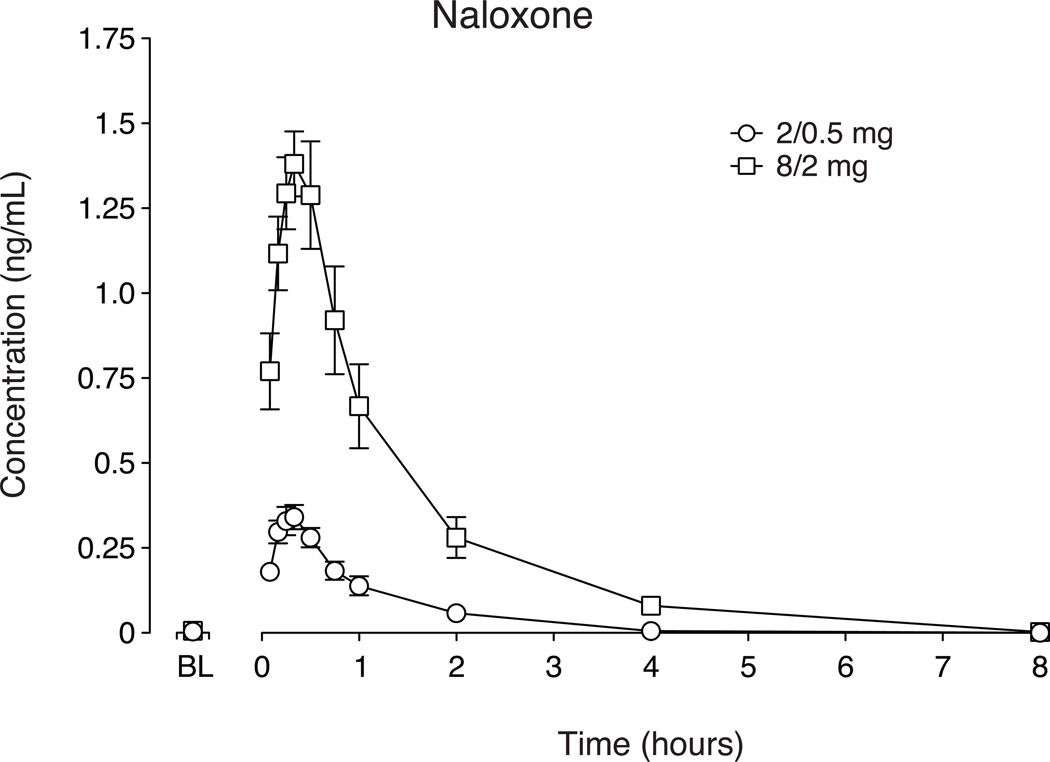
Naloxone plasma concentrations after intranasal buprenorphine/naloxone (Fig. 5; Table 3) increased dose-dependently (P < 0.0001). Plasma naloxone concentration returned to baseline levels (<LLOQ) within 8 hours following drug administration (with the exception of one session in which naloxone concentration was 0.025 ng/ml, the LLOQ for naloxone). Significant differences between 2/0.5 and 8/2 mg buprenorphine/naloxone were observed for naloxone bioavailability, t1/2, AUC and Cmax (P < 0.05) but not Tmax.
Figure 5.
Data illustrate mean (n=10) values ± standard error of the mean for plasma concentrations of naloxone. Plasma concentrations of naloxone were analyzed for up to 8 hours after drug administration.
Safety
No serious adverse events occurred. Side effects reported/observed after active drug administration included vomiting during (n=4) and after (n=5) a test session, constipation (n=5), and headache during (n=4; three during an active drug session and one after placebo) and after (n=7; five after an active drug session and two after placebo) a test session. One subject reported blurry vision during session after an active dose.
DISCUSSION
This study examined the intranasal pharmacodynamic and pharmacokinetic profile of crushed buprenorphine and buprenorphine/naloxone tablets among sporadic opioid users. This study employed crushed buprenorphine and buprenorphine/naloxone tablets, which is important because these are the tablets used to treat opioid dependence and for which there are reports of intranasal misuse. Both formulations were safely tolerated (with minimal effects on oxygen saturation and respiratory rate) and produced dose-dependent prototypic subjective and physiological mu opioid effects. Overall, there were few statistical differences between buprenorphine alone and buprenorphine/naloxone; however, subjects reported the highest ratings of positive mood effects (‘like’, ‘high’, ‘drug effect’, ‘good effects’) and street values for 8 mg of buprenorphine. Physiological and subjective time course data reveal a modestly slower onset of action for 8/2 mg buprenorphine/naloxone compared to 8 mg buprenorphine alone, and subjects were able to differentiate between formulations based on medication taste when snorted. Buprenorphine was well absorbed intranasally with no differences between formulations in time course, Cmax of the parent drug or metabolites. Naloxone was readily absorbed after intranasal buprenorphine/naloxone administration with an estimated bioavailability between 24 and 30%.
Consistent with earlier studies of sublingual buprenorphine, with and without naloxone [8, 11, 19, 20, 38, 39], intranasal administration increased subjective ratings significantly on abuse liability measures in non-dependent opioid abusers. Moreover, the peak ratings for visual analog indices of ‘high’ and ‘liking’ and pupillary miosis observed here after intranasal doses of 8 mg were comparable in magnitude to peak responses after sublingual administration of that same dose [8, 19]. The onset of pharmacodynamic effects was modestly, but not significantly, slower for buprenorphine/naloxone compared to buprenorphine alone, a pattern previously reported after intramuscular administration of the single and combination products [20]. Importantly, intranasal administration of buprenorphine did differ significantly from sublingual dosing with regard to the speed of drug onset and the time-required-to-reach the maximum effect, both factors known to influence abuse liability. In the present study, the onset of drug action was evident within 15 minutes of drug administration and peak pharmacodynamic responses were achieved on average between 60 and 75 minutes after dosing. In contrast, studies of sublingual buprenorphine have reported a slower onset of action (30–45 minutes) and peak pharmacodynamic responses occurring between 2 and 3 hours after dosing [8, 19]. Buprenorphine concentrations following intranasal administration of the high doses of buprenorphine and buprenorphine/naloxone in the current study were approximately 2–3 fold higher than studies of sublingual dosing [30, 31, 40, 41] and peak plasma concentrations were reached at least 30 minutes earlier compared to the Tmax for sublingual administration. Thus, it is possible that opioid abusers are misusing buprenorphine by the intranasal route to attain higher concentrations more rapidly than if buprenorphine is taken as prescribed.
When administered as a sublingual tablet, bioavailability of buprenorphine can be as low as 15% [30], while a study of an intranasal buprenorphine solution reported bioavailability at 48% [42]. In the current study, the bioavailability of intranasal buprenorphine from crushed tablets ranged between 38 and 44%. Naloxone did not alter the pharmacokinetic action of buprenorphine or its metabolites. When taken sublingually, naloxone has poor absorption and bioavailability (16). To our knowledge, only one study by Dowling and colleagues has examined naloxone absorption following intranasal administration in humans and determined that naloxone was detectable in only 1/3 of the subjects receiving 2 mg naloxone (in solution) and was not detectable following 0.8 mg [43]. The same study also determined that intranasal naloxone had poor intranasal bioavailability (i.e. 4%). The current study determined that naloxone bioavailability was 24% and 30% for doses containing 0.5 and 2 mg naloxone, respectively. Dowling et al. [43] administered 0.5 ml of naloxone through a cannula and flushed with 5 ml of saline. For that study, the solution volume exceeded the nasal mucosa limit for absorption (i.e., 0.3–0.4 ml; [44]); thus, excess solution may have been swallowed decreasing the naloxone available for intranasal absorption. Also, nasal inhalation of powder versus solution may result in differential absorption characteristics. Previous studies in rats and rabbits have shown increased absorption of insulin following administration of powder drug formulation compared to liquid formulation [45, 46].
Based on the current results, it is not evident that the observed differences with regard to abuse potential between the two formulations are clinically relevant at the doses tested here. It is important to note that the doses tested here are in the low range of therapeutic use, and persons misusing drugs often misuse higher doses. It is possible that intranasal administration of higher doses (e.g., 16 or 24 mg) may show differences between formulations, as naloxone is clearly bioavailable. Administration of low doses of naloxone (0.1–0.5 mg, intravenously or intramuscularly) will precipitate withdrawal in patients who are dependent on most opioids [16–18, 47–58]. In the current study, bioavailability and Cmax of intranasal naloxone were approximately three- and five-times greater, respectively, compared to sublingual naloxone studies [16, 27, 40]. Given the plasma concentrations and bioavailability of naloxone observed in this study, it is likely (but has not yet been reported) that intranasal use of buprenorphine/naloxone may lead to precipitated withdrawal in opioid dependent individuals.
Epidemiological and post-marketing surveillance studies have shown that buprenorphine is misused by the intranasal route [24–26, 59]. The greater bioavailability (i.e., higher plasma concentrations) and faster onset of pharmacodynamic effects following intranasal compared to sublingual administration explains the motivation for misuse by this route in non-dependent opioid abusers. However, the significant absorption of naloxone from intranasal buprenorphine/naloxone administration observed here may deter the likelihood of intranasal misuse of buprenorphine/naloxone, but not the buprenorphine monoproduct, in some opioid dependent individuals.
Acknowledgements
The authors thank Pamela Henderson, Rachel Ridd, Victoria Vessels and Wenfang Fang for their technical research support, Stephen Sitzlar PharmD for pharmacy services, and Martha Wunsch MD, Marie Bate RN and Stacy Miller RN for medical support and consultation. The authors are indebted to the UK Clinical Research Development and Operations Center for in-patient services, supervision and nutritional services. We thank Research Triangle Institute, the NIDA Drug Supply Program and Reckitt Benckiser Pharmaceuticals for provision of study drug without cost. We also thank Dr. Chris Chapleo for facilitating Reckitt Benckiser’s support of the plasma analyses through a contract with the University of Utah (D.E.M.). This project was supported by the National Institute on Drug Abuse (R01-DA016718; S.L.W. and T32 DA016176; L.S.M.) and a contract to support the plasma drug analysis from Reckitt Benckiser Pharmaceuticals to the University of Utah (D.E.M.).
Footnotes
Declaration of Interest: Dr. Michelle Lofwall has received speaker honoraria and is the recipient of an investigator-initiated educational research contract from Reckitt Benckiser Pharmaceuticals, a manufacturer of buprenorphine and buprenorphine/naloxone. Dr. David Moody has received research funding (including contractual support for the pharmacokinetic analyses performed for this study) and consultation fees from Reckitt Benckiser Pharmaceuticals. Dr. Sharon Walsh has received travel reimbursement and honoraria from Reckitt Benckiser Pharmaceuticals as a speaker and consultant.
References
- 1.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–2755. [PubMed] [Google Scholar]
- 2.Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P, et al. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 3.Auriacombe M, Fatseas M, Dubernet J, Daulouede JP, Tignol J. French field experience with buprenorphine. Am J Addict. 2004;13:S17–S28. doi: 10.1080/10550490490440780. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell JC, McCance-Katz EF. Indicators of buprenorphine and methadone use and abuse: what do we know? Am J Addict. 2010;19:73–88. doi: 10.1111/j.1521-0391.2009.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta N, Bailey EJ, Cicero T, Inciardi J, Parrino M, Rosenblum A, et al. Post-marketing surveillance of methadone and buprenorphine in the United States. Pain Med. 2010;11:1078–1091. doi: 10.1111/j.1526-4637.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- 6.Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 9.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94:825–834. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 10.Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- 12.Comer SD, Sullivan MA, Vosburg SK, Manubay J, Amass L, Cooper ZD, et al. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010;105:709–718. doi: 10.1111/j.1360-0443.2009.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal A, Tripathi BM, Pal HR, Jena R, Jain R. Subjective effects of additional doses of buprenorphine in patients on buprenorphine maintenance. Addict Behav. 2007;32:320–331. doi: 10.1016/j.addbeh.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology (Berl) 1995;119:268–276. doi: 10.1007/BF02246290. [DOI] [PubMed] [Google Scholar]
- 15.Cicero TJ, Surratt HL, Inciardi J. Use and misuse of buprenorphine in the management of opioid addiction. J Opioid Manag. 2007;3:302–308. doi: 10.5055/jom.2007.0018. [DOI] [PubMed] [Google Scholar]
- 16.Preston KL, Bigelow GE, Liebson IA. Effects of sublingually given naloxone in opioid-dependent human volunteers. Drug Alcohol Depend. 1990;25:27–34. doi: 10.1016/0376-8716(90)90136-3. [DOI] [PubMed] [Google Scholar]
- 17.Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl) 2001;154:230–242. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- 18.Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN. Effects of buprenorphine and naloxone in morphine-stabilized opioid addicts. Drug Alcohol Depend. 1998;50:1–8. doi: 10.1016/s0376-8716(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Strain EC, Stoller K, Walsh SL, Bigelow GE. Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology (Berl) 2000;148:374–383. doi: 10.1007/s002130050066. [DOI] [PubMed] [Google Scholar]
- 20.Weinhold LL, Preston KL, Farre M, Liebson IA, Bigelow GE. Buprenorphine alone and in combination with naloxone in non-dependent humans. Drug Alcohol Depend. 1992;30:263–274. doi: 10.1016/0376-8716(92)90061-g. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- 22.Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Degenhardt L, Larance BK, Bell JR, Winstock AR, Lintzeris N, Ali RL, et al. Injection of medications used in opioid substitution treatment in Australia after the introduction of a mixed partial agonist-antagonist formulation. Med J Aust. 2009;191:161–165. doi: 10.5694/j.1326-5377.2009.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Justice. Intelligence Bulletin: Buprenorphine: Potential for Abuse. [Accessed January 11, 2011];2004 [updated 2004]; Available at: http://www.justice.gov/ndic/pubs10/10123/ Archived by WebCite® at http://www.webcitation.org/5vdOJEP0j.
- 25.Barrau K, Thirion X, Micallef J, Chuniaud-Louche C, Bellemin B, San Marco JL. Comparison of methadone and high dosage buprenorphine users in French care centres. Addiction. 2001;96:1433–1441. doi: 10.1046/j.1360-0443.2001.961014337.x. [DOI] [PubMed] [Google Scholar]
- 26.Roux P, Villes V, Bry D, Spire B, Feroni I, Marcellin F, et al. Buprenorphine sniffing as a response to inadequate care in substituted patients: results from the Subazur survey in south-eastern France. Addict Behav. 2008;33:1625–1629. doi: 10.1016/j.addbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Harris DS, Jones RT, Welm S, Upton RA, Lin E, Mendelson J. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend. 2000;61:85–94. doi: 10.1016/s0376-8716(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 28.Kuhlman JJ, Jr, Lalani S, Magluilo J, Jr, Levine B, Darwin WD. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20:369–378. doi: 10.1093/jat/20.6.369. [DOI] [PubMed] [Google Scholar]
- 29.Mendelson J, Upton RA, Everhart ET, Jacob P, 3rd, Jones RT. Bioavailability of sublingual buprenorphine. J Clin Pharmacol. 1997;37:31–37. doi: 10.1177/009127009703700106. [DOI] [PubMed] [Google Scholar]
- 30.Nath RP, Upton RA, Everhart ET, Cheung P, Shwonek P, Jones RT, et al. Buprenorphine pharmacokinetics: relative bioavailability of sublingual tablet and liquid formulations. J Clin Pharmacol. 1999;39:619–623. doi: 10.1177/00912709922008236. [DOI] [PubMed] [Google Scholar]
- 31.Schuh KJ, Johanson CE. Pharmacokinetic comparison of the buprenorphine sublingual liquid and tablet. Drug Alcohol Depend. 1999;56:55–60. doi: 10.1016/s0376-8716(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 32.Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GE. Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug Alcohol Depend. 2004;74:37–43. doi: 10.1016/j.drugalcdep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 35.Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) "Attitude" of opiate addicts toward opiate-like drugs. (B) a short-term "direct" addiction test. J Pharmacol Exp Ther. 1961;133:371–387. [PubMed] [Google Scholar]
- 36.McLeod D, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–466. [Google Scholar]
- 37.Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 38.Bedi NS, Ray R, Jain R, Dhar NK. Abuse liability of buprenorphine--a study among experienced drug users. Indian J Physiol Pharmacol. 1998;42:95–100. [PubMed] [Google Scholar]
- 39.Duke AN, Correia CJ, Walsh SL, Bigelow GE, Strain EC. Acute effects of intramuscular and sublingual buprenorphine and buprenorphine/naloxone in non-dependent opioid abusers. Psychopharmacology (Berl) 2010;211:303–312. doi: 10.1007/s00213-010-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciraulo DA, Hitzemann RJ, Somoza E, Knapp CM, Rotrosen J, Sarid-Segal O, et al. Pharmacokinetics and pharmacodynamics of multiple sublingual buprenorphine tablets in dose-escalation trials. J Clin Pharmacol. 2006;46:179–192. doi: 10.1177/0091270005284192. [DOI] [PubMed] [Google Scholar]
- 41.Harris DS, Mendelson JE, Lin ET, Upton RA, Jones RT. Pharmacokinetics and subjective effects of sublingual buprenorphine, alone or in combination with naloxone: lack of dose proportionality. Clin Pharmacokinet. 2004;43:329–340. doi: 10.2165/00003088-200443050-00005. [DOI] [PubMed] [Google Scholar]
- 42.Eriksen J, Jensen NH, Kamp-Jensen M, Bjarno H, Friis P, Brewster D. The systemic availability of buprenorphine administered by nasal spray. J Pharm Pharmacol. 1989;41:803–805. doi: 10.1111/j.2042-7158.1989.tb06374.x. [DOI] [PubMed] [Google Scholar]
- 43.Dowling J, Isbister GK, Kirkpatrick CM, Naidoo D, Graudins A. Population pharmacokinetics of intravenous, intramuscular, and intranasal naloxone in human volunteers. Ther Drug Monit. 2008;30:490–496. doi: 10.1097/FTD.0b013e3181816214. [DOI] [PubMed] [Google Scholar]
- 44.Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57:1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Hussain A, Majumder QH, Ahsan F. Inhaled insulin is better absorbed when administered as a dry powder compared to solution in the presence or absence of alkylglycosides. Pharm Res. 2006;23:138–147. doi: 10.1007/s11095-005-8926-9. [DOI] [PubMed] [Google Scholar]
- 46.Schipper NG, Romeijn SG, Verhoef JC, Merkus FW. Nasal insulin delivery with dimethyl-beta-cyclodextrin as an absorption enhancer in rabbits: powder more effective than liquid formulations. Pharm Res. 1993;10:682–686. doi: 10.1023/a:1018999414088. [DOI] [PubMed] [Google Scholar]
- 47.Strain EC, Preston KL, Liebson IA, Bigelow GE. Acute effects of buprenorphine, hydromorphone and naloxone in methadone-maintained volunteers. J Pharmacol Exp Ther. 1992;261:985–993. [PubMed] [Google Scholar]
- 48.Strain EC, Preston KL, Liebson IA, Bigelow GE. Precipitated withdrawal by pentazocine in methadone-maintained volunteers. J Pharmacol Exp Ther. 1993;267:624–634. [PubMed] [Google Scholar]
- 49.Preston KL, Bigelow GE, Liebson IA. Buprenorphine and naloxone alone and in combination in opioid-dependent humans. Psychopharmacology (Berl) 1988;94:484–490. doi: 10.1007/BF00212842. [DOI] [PubMed] [Google Scholar]
- 50.Preston KL, Bigelow GE, Liebson IA. Antagonist effects of nalbuphine in opioid-dependent human volunteers. J Pharmacol Exp Ther. 1989;248:929–937. [PubMed] [Google Scholar]
- 51.Kanof PD, Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Rubinstein KJ. Clinical characteristics of naloxone-precipitated withdrawal in human opioid-dependent subjects. J Pharmacol Exp Ther. 1992;260:355–363. [PubMed] [Google Scholar]
- 52.Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML. Buprenorphine's physical dependence potential: antagonist-precipitated withdrawal in humans. J Pharmacol Exp Ther. 1996;276:449–459. [PubMed] [Google Scholar]
- 53.Kosten TR, Krystal JH, Charney DS, Price LH, Morgan CH, Kleber HD. Opioid antagonist challenges in buprenorphine maintained patients. Drug Alcohol Depend. 1990;25:73–78. doi: 10.1016/0376-8716(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 54.Mendelson J, Jones RT, Welm S, Baggott M, Fernandez I, Melby AK, et al. Buprenorphine and naloxone combinations: the effects of three dose ratios in morphine-stabilized, opiate-dependent volunteers. Psychopharmacology (Berl) 1999;141:37–46. doi: 10.1007/s002130050804. [DOI] [PubMed] [Google Scholar]
- 55.Mendelson J, Jones RT, Welm S, Brown J, Batki SL. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095–1101. doi: 10.1016/S0006-3223(96)00266-1. [DOI] [PubMed] [Google Scholar]
- 56.Nigam AK, Srivastava RP, Saxena S, Chavan BS, Sundaram KR. Naloxone-induced withdrawal in patients with buprenorphine dependence. Addiction. 1994;89:317–320. doi: 10.1111/j.1360-0443.1994.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 57.Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100mg of daily methadone. Drug Alcohol Depend. 2007;90:261–269. doi: 10.1016/j.drugalcdep.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuh KJ, Walsh SL, Bigelow GE, Preston KL, Stitzer ML. Buprenorphine, morphine and naloxone effects during ascending morphine maintenance in humans. J Pharmacol Exp Ther. 1996;278:836–846. [PubMed] [Google Scholar]
- 59.Strang J. Abuse of buprenorphine (Temgesic) by snorting. BMJ. 1991;302:969. doi: 10.1136/bmj.302.6782.969-b. [DOI] [PMC free article] [PubMed] [Google Scholar]