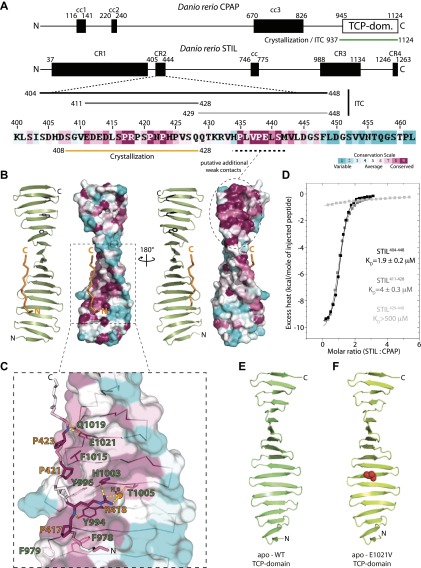
Figure 1. Biochemical and structural characterisation of the CPAP TCP domain and its interaction with STIL.
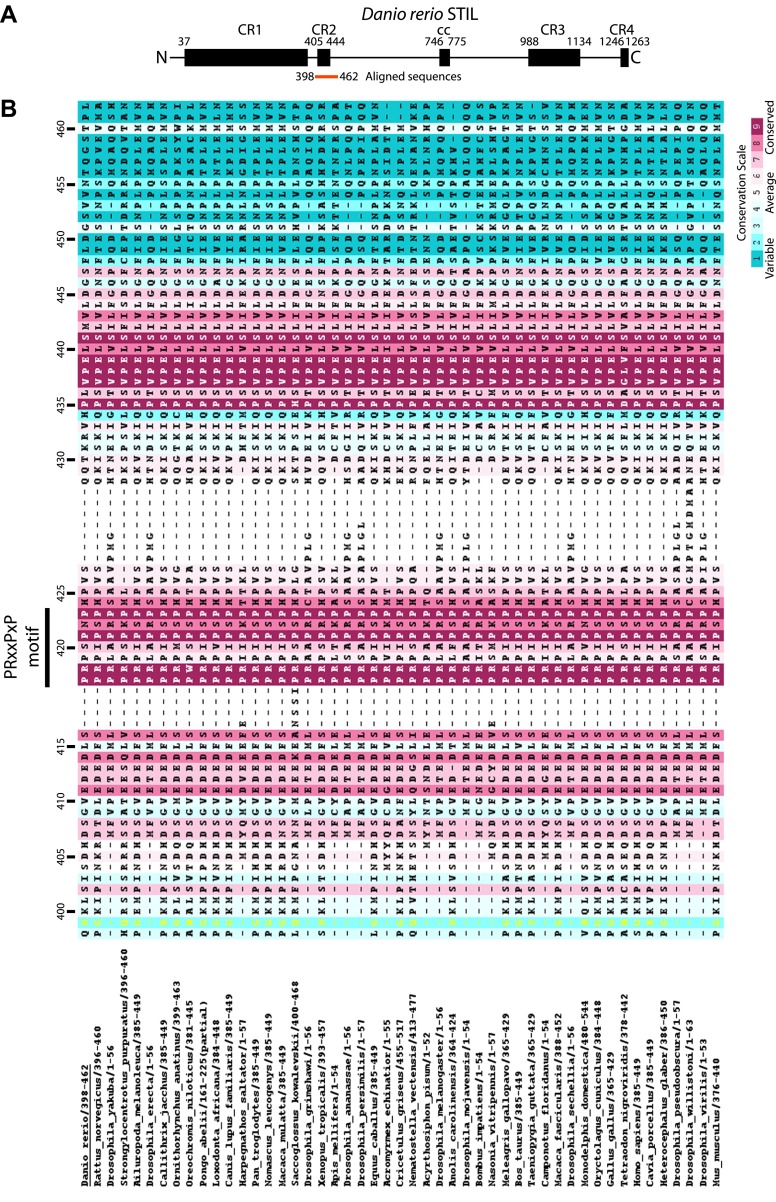
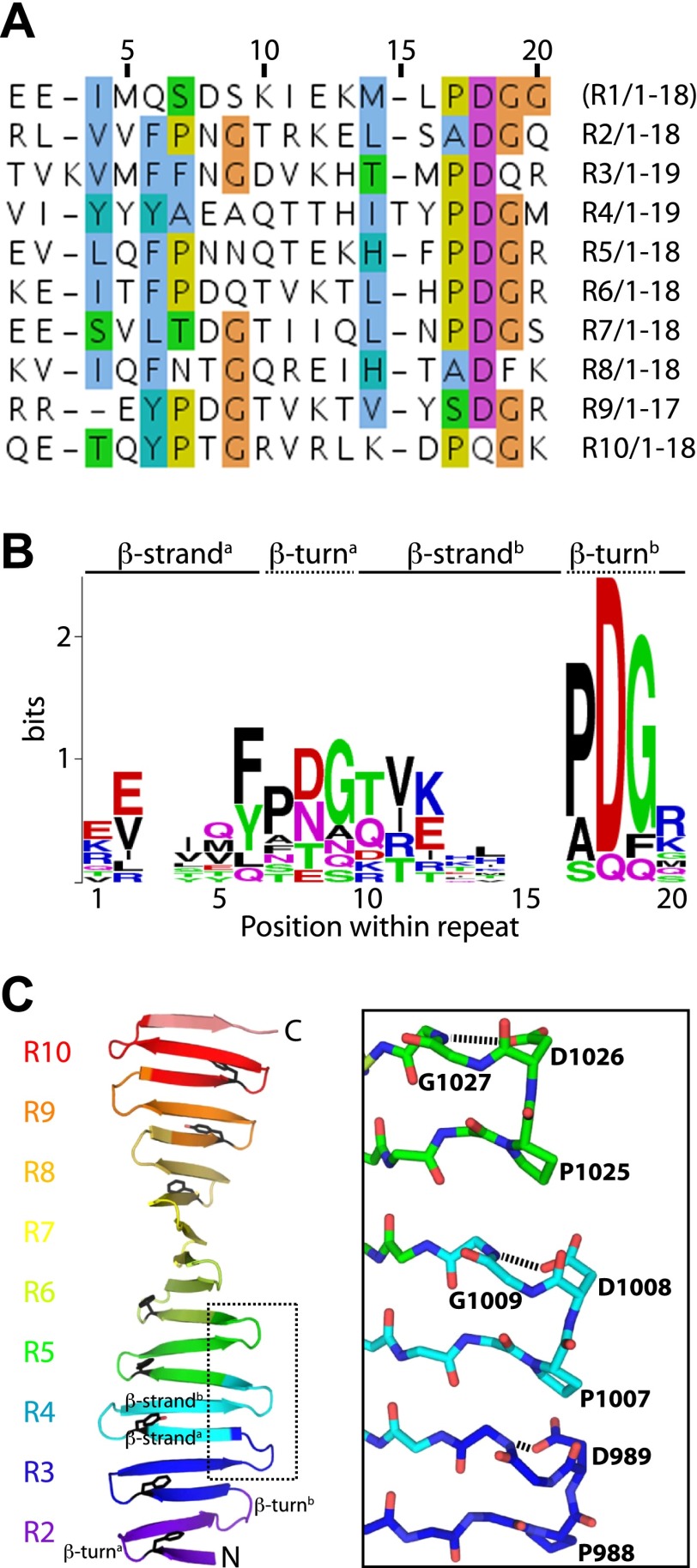
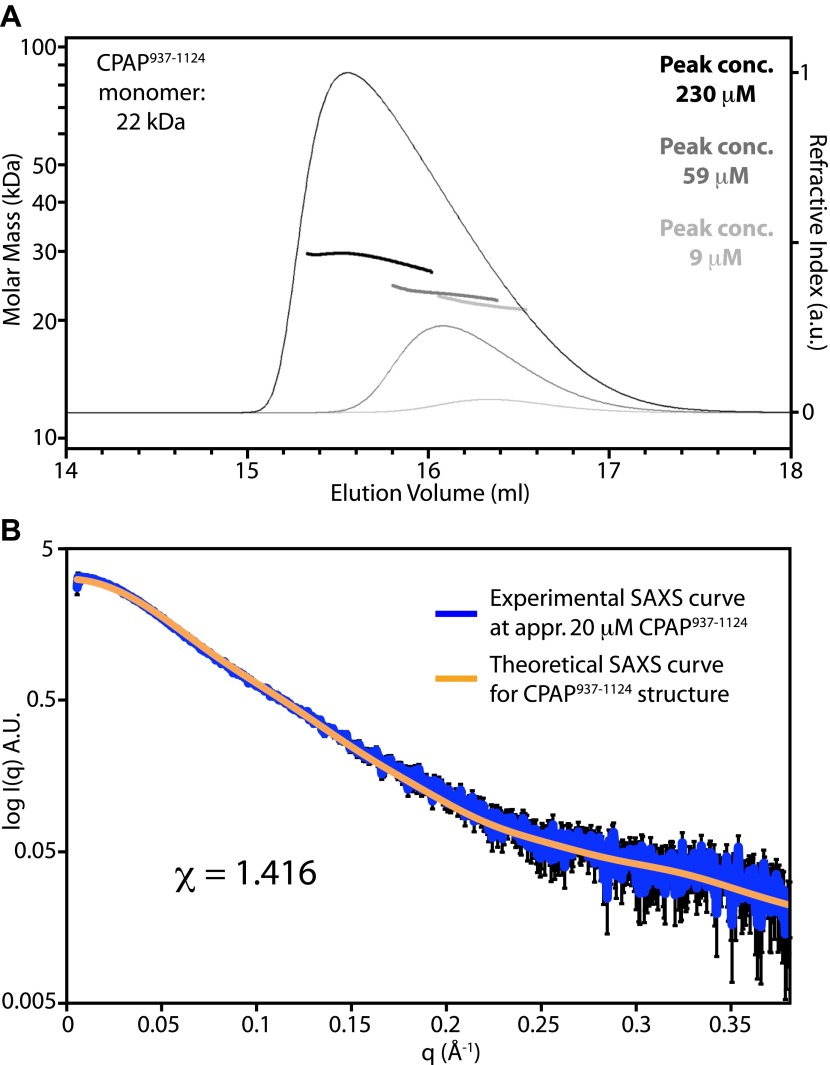
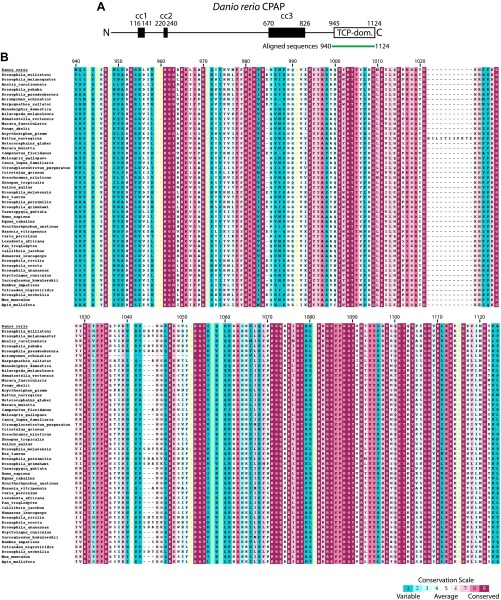
(A) Schematic representation of D. rerio CPAP and STIL. CPAP is a 1124 amino acid (aa) protein with three predicted coiled coil (cc) domains and a C-terminal TCP domain. STIL is a 1263 aa protein with one predicted cc domain and several conserved regions (CR). The proline-rich CR2 domain is enlarged and coloured according to Consurf conservation scores (Glaser et al., 2003) from cyan (variable) to burgundy (conserved). The constructs used in this study are indicated by bars. (B) Two views of the TCP domain structure (green) in complex with the STIL peptide (orange), rotated by 180°. Images on the left of each view show a ribbon representation and images on the right show the TCP domain as a molecular surface coloured according to Consurf conservation scores. Note the presence of a conserved patch (dashed circle) along the edge of the TCP domain where the STIL peptide is bound. This patch contains aromatic residues (black sticks) that would be well placed to interact with conserved prolines in the C-terminal part of the STIL CR2 region that we had to omit for crystallisation. ITC experiments (Figure 1D) suggest that these putative additional contacts would only contribute weakly to overall binding. (C) Detailed view of the D. rerio CPAP–STIL interaction interface coloured according to Consurf conservation scores. Interface residues are shown in sticks, and the TCP domain is shown as a semi-transparent molecular surface. Contact residues are labelled in green (CPAP) and orange (STIL). Dotted yellow lines indicate hydrogen-bonds. The dark orange sphere represents a bound water molecule. (D) ITC analysis using the STIL constructs shown in Figure 1A. The excess heat measured on titrating STIL into CPAP at 25°C was fitted to a single set of binding sites model. Fitted KD values are indicated together with their standard deviations. (E and F) Ribbon models of the apo-structures of the D. rerio CPAP TCP domain: (E) WT apo-structure; (F) E1021V (MCPH mutation) apo-structure (V1021 represented as red spheres).