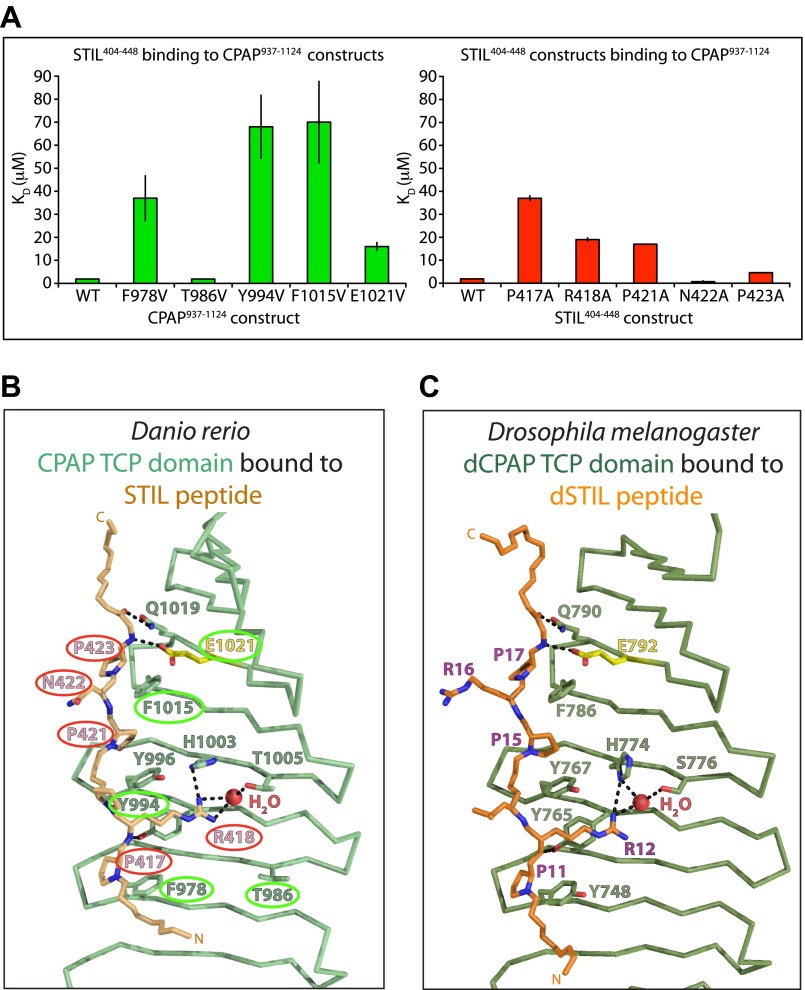
Figure 2. Mutational analysis of the CPAP:STIL interaction in vitro and conservation of the interaction across species.
(A) Graphs showing the binding constants (KD) determined by ITC for the interaction between WT and mutant constructs of CPAP937–1124 and STIL404–448. Left panel, WT and various mutant forms of CPAP937–1124 binding to WT STIL404–448 (T986 is a non-interacting residue included as a negative control). Error bars, standard deviation. Right panel, WT and various mutant STIL404–448 constructs binding to WT CPAP937–1124 (N422 is a non-interacting residue included as a negative control). Error bars, standard deviation. The wild-type measurements are the same as shown in Figure 1D and are shown again for comparison to the mutants. (B and C) Close-up view of the CPAP (green):STIL (orange) interaction interface from D. rerio (B) and Drosophila (C). Interface residues are shown as sticks, in yellow is the Glutamate residue in Drosophila and D. rerio CPAP that is equivalent to E1235 in human CPAP (mutated in MCPH). Residues of the D. rerio protein mutated for ITC experiments are ringed in green (CPAP) or red (STIL). Dotted black lines indicate hydrogen-bonds. The conserved bound water molecule is shown as a red sphere.