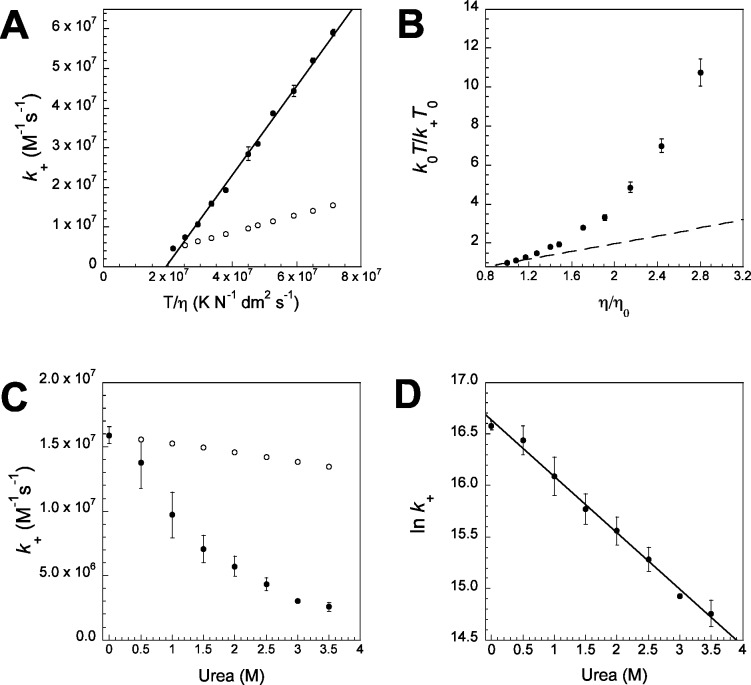
Figure 3.
(A) The experimental association rate constant k+ (●) is not directly proportional to T/η, as would be predicted for a ‘diffusion-limited’ reaction according to eq 2 (○, arbitrarily drawn through the 10 °C data point). Where repeat measurements were made, standard errors are shown as error bars. (B) Temperature corrected viscosity plot shows clear nonlinearity, in contrast to what is expected for a ‘diffusion-limited’ reaction (gradient of 1 shown as a dashed line). (C) k+ is reduced by increasing the concentration of the denaturant urea (●) and this effect is not accounted for by the slower diffusion due to changes in solvent viscosity (k+ = k0η0/η, ○). (D) The log of the rate constant is linearly dependent on urea concentration, suggesting that a structured state is being energetically disfavored.