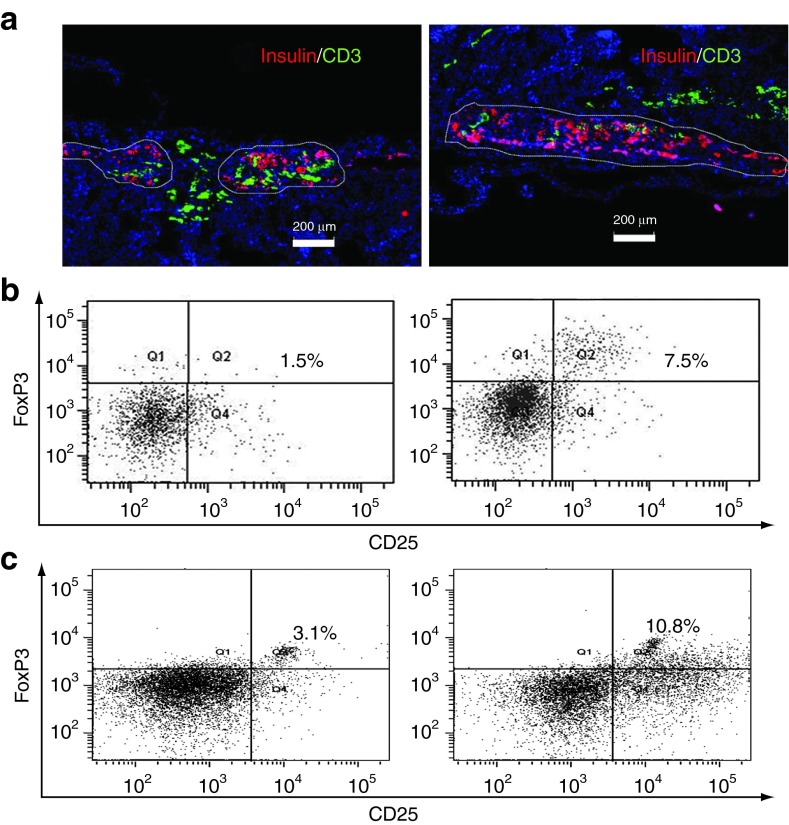
Figure 5.
Human bone marrow-derived mesenchymal stem cells (hBMSCs) prevented T cell infiltration into the transplantation site. (a) The immune fluorescence staining of the kidney section bearing human islets alone (left) or with hBMSCs (right). Briefly, three mice in each group were killed at 2 weeks after the injection of peripheral blood mononuclear cells (PBMCs, 5 × 106/mice), or 6 weeks after islet transplantation. Insulin was stained in red to identify the transplanted human islets, while CD3+ T cells were stained in green. The white outlines indicated the boundary of functional islets. The slides were counter-stained by 4′,6-diamidino-2-phenylindole (blue) which passed through membrane and stained the nuclei specifically. Scale bar represents 200 µm. All experiments were done in triplicates and a representative picture was shown. (b) The percentage of CD4+CD25+FoxP3+ regulatory T cells (Tregs) recovered from the blood of NOD scid gamma (NSG) mice at 2 weeks after the injection of PBMCs. Briefly, CD4+ T cells were isolated from the total blood of NSG mice using Dynabeads at the end of study. The percentage of CD25+FoxP3+ Tregs among the total CD4+ T cells was determined by flow cytometry. Q2 represents the Tregs. (c) The percentage of Tregs of the total CD4+ T cells recovered from PBMC/hBMSC coculture. Briefly, PBMCs (1 × 106) were stimulated with phytohemagglutinin (1 µg/ml) for 24 hours and cocultured with hBMSCs (5 × 104) in a transwell for 5 more days. Total CD4+ T cells were isolated using Dynabeads at the end of the study. The CD4+CD25+FoxP3+ Tregs were stained using Human Regulatory T cells 3-color kit and the percentage of Tregs in the total CD4+ T cells was calculated using flow cytometry.