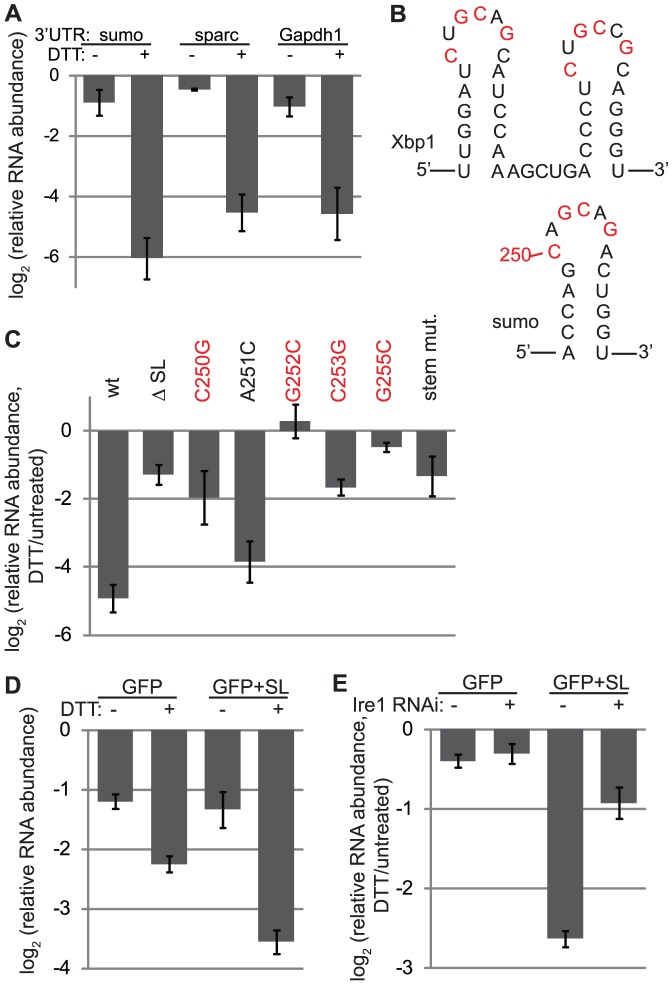
Figure 2. A stem loop sequence in the sumo mRNA is important for RIDD targeting.
For panels A, C-E: plasmids expressing reporter mRNAs under the control of a copper-inducible promoter were stably transfected into S2 cells. After inducing expression, we removed the copper to stop transcription of reporter mRNAs, incubated cells in the presence and absence of ER stress (2 mM DTT, 5 hrs), and collected RNA samples. Relative RNA abundance was measured by qPCR and normalized to Rpl19. Shown are the averages and SDs of 3 (panels A, C) or 2 (panels D-E) independent experiments. A. Reporters expressing the coding sequence of sumo followed by various 3′UTRs. We normalized RNA levels to a control sample collected immediately before washing out the copper; thus RNA measurements reflect the amount of degradation after 5 hrs without copper. B. RNA sequences of sumo and Xbp1 from D. melanogaster, surrounding the stem loop structures discussed here. Highlighted in red are the loop nucleotides conserved in Xbp1 across species. Numbering in the sumo mRNA is relative to the translation start site. C. Reporters containing the sumo coding sequence and 3′ UTR, with various mutations. ΔSL = deletion of nucleotides 244–270 in the coding sequence of sumo; stem mut. = C257G/G259C/G260C. D. Reporters expressing GFP with the Gapdh1 3′UTR, with and without the stemloop sequence of sumo (nt 244–270). E. Degradation of reporters from D in untreated cells and those depleted of Ire1.