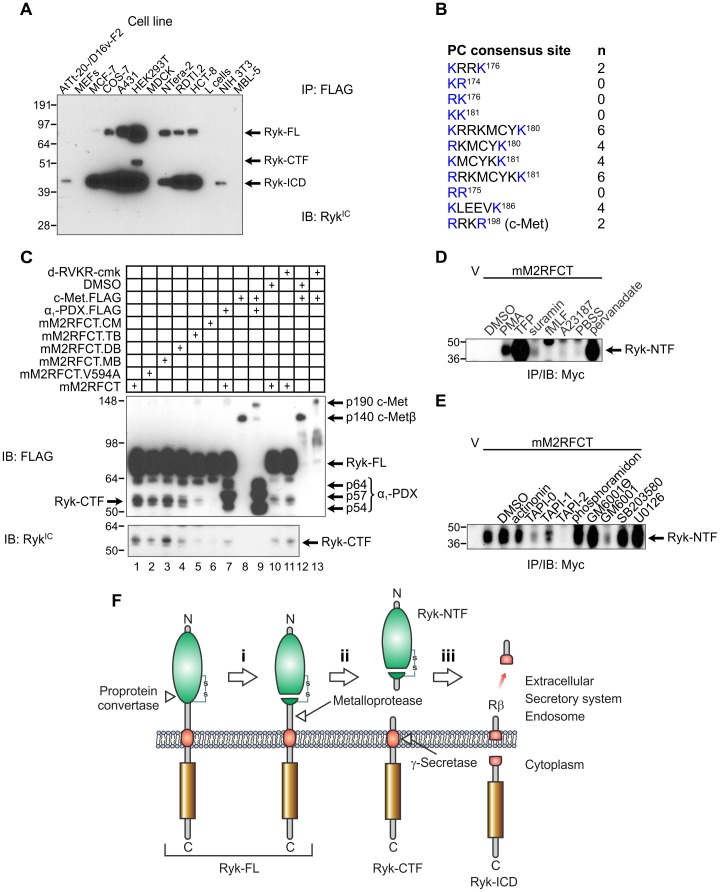
Figure 2. Proteolytic processing of the mouse Ryk extracellular region.
(A) Proteolysis of Ryk in mammalian cell lines. Cells were transiently transfected with pcDNA3.mM2RFCT and lysed 48 h later. Anti-FLAG immunoprecipitates (IP) were immunoblotted (IB) with rabbit anti-RykIC polyclonal antibody. Molecular mass standards are shown at left in kDa. MEFs, mouse embryonic fibroblasts; RDTI.2, Ryk-deficient large T antigen-immortalized fibroblasts derived from a Ryk −/− embryo. (B) Consensus cleavage sites in the mouse Ryk extracellular region for PC1, PC2, furin, PC4, PC5, PACE4 and PC7 (single-letter amino acid code; basic residues conforming to the consensus in blue; residues numbered according to NCBI Reference Sequence NP_038677.3; (K/R)Xn(K/R)↓, where X is any residue, n = 0, 2, 4 or 6 and the downward arrow represents cleavage [53]). The furin cleavage site in the mouse c-Met extracellular domain is shown for comparison. (C) COS-7 cells were transiently transfected with plasmids encoding mM2RFCT or the derivatives V594A; K186Q (monobasic, MB); KK181→QQ181 (dibasic, DB); KRRK176→QQQQ176 (tetrabasic, TB); and QQQQ176;QQ181;Q186 (compound mutant; CM). The α1-PDX.FLAG protein (p54, p57 and p64 isoforms indicated) was expressed to inhibit endogenous furin. Mouse c-Met.FLAG was expressed as a positive control for inhibition of furin. The location and identity of Ryk-CTF was confirmed using anti-RykIC polyclonal antibody (bottom panel). Molecular mass standards are shown at left in kDa. (D) Transiently transfected COS-7 cells were treated with potential activators of receptor shedding for 30 min. Anti-Myc IPs were prepared from the conditioned medium and immunoblotted with an anti-Myc antibody. Molecular mass standards are shown at left in kDa. V, empty vector (pcDNA3)-transfected cells; PBSS, phosphate-buffered saline with calcium and magnesium used as diluent for pervanadate. (E) Transiently transfected COS-7 cells were pre-treated with protease inhibitors, then shedding was activated with TFP (100 µM, 30 min). Anti-Myc IPs were analyzed as in (C). Molecular mass standards are shown at left in kDa. (F) Model for sequential proteolysis of Ryk. The metalloprotease-mediated cleavage in step (ii) has constitutive and inducible components. Green, WIF domain; gold, PTK domain; red, transmembrane helix; Ryk-FL, full-length uncleaved Ryk; Ryk-CTF, Ryk carboxyl-terminal fragment; Ryk-ICD, Ryk intracellular domain fragment; Rβ, predicted membrane-associated peptide analogous to amyloid-β peptide.