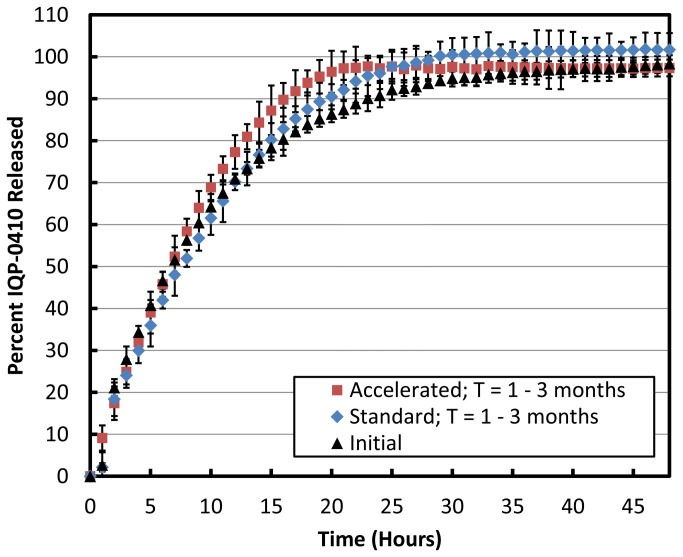
Figure 2. In vitro release for IQP-0410 from transdermal films.
Under 10:90 EtOH-Water sink conditions, the in vitro release of IQP-0410 from the transdermal films were evaluated in a USP-4 apparatus for 48 hours upon initial manufacturing (black triangles) (n = 3 ± SD). The transdermal films were packaged into air-tight foil pouches and stored under standard (30°C/65% R.H.) and accelerated (40°C/75% R.H.) environmental conditions for 3 months. At 1 month, 2 months, and 3 months, the transdermal films were removed from storage and tested for drug release. The combined average (n = 9 ± SD) of the transdermal films stored under standard environmental conditions for 3 months (blue diamonds). The combined average (n = 9 ± SD) of the transdermal films stored under accelerated environmental conditions for 3 months (red squares).