Figure 5. CL4 binding leads to similar chemical shift changes for a CL4-NBD1 fusion protein.
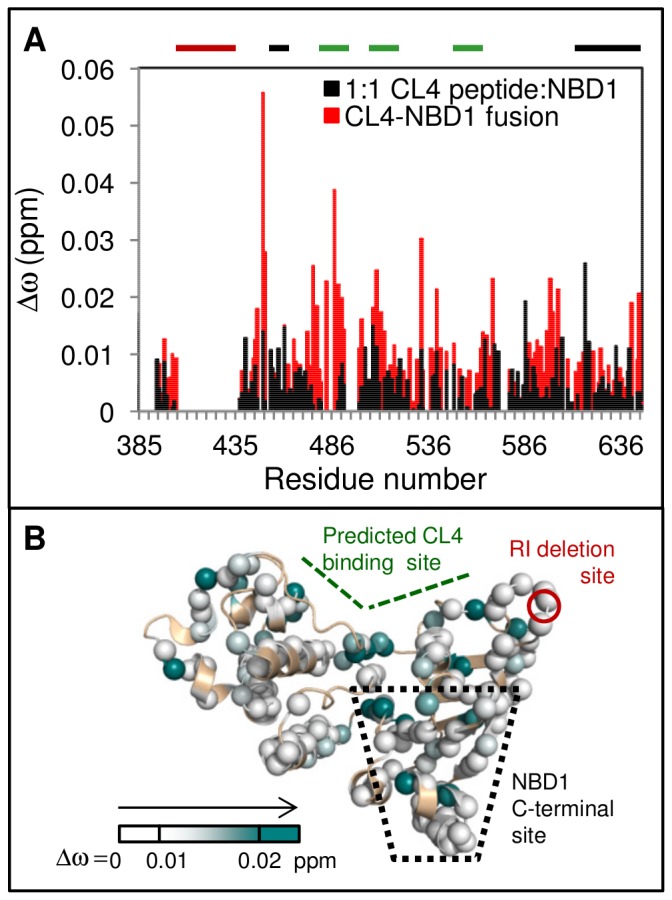
A. Chemical shift differences, Δωobs, between the CL4-NBD1 fusion protein and NBD1(red) overlaid with Δωobs for 1∶1 CL4 peptide:NBD1 (open black bars). There is an increase in Δωobs in the CL4-binding and NBD1 C-terminal sites for the fusion protein, compared to the assay of the isolated reagents. The CL4-binding site, the RI residues deleted from the construct and the NBD1 C-terminal site are indicated with thick bars above the chart that are colored green, red, and black, respectively. B. CL4-NBD1 Δωobs mapped onto NBD1 structure. A shallower white-to-teal gradient was used to map the Δωobs values than was used, reflecting the smaller chemical shifts changes observed for intra-molecular CL4 binding within the fusion protein as compared to those for isolated NBD1 in the presence of a 12.5∶1 excess of CL4 peptide.
