Abstract
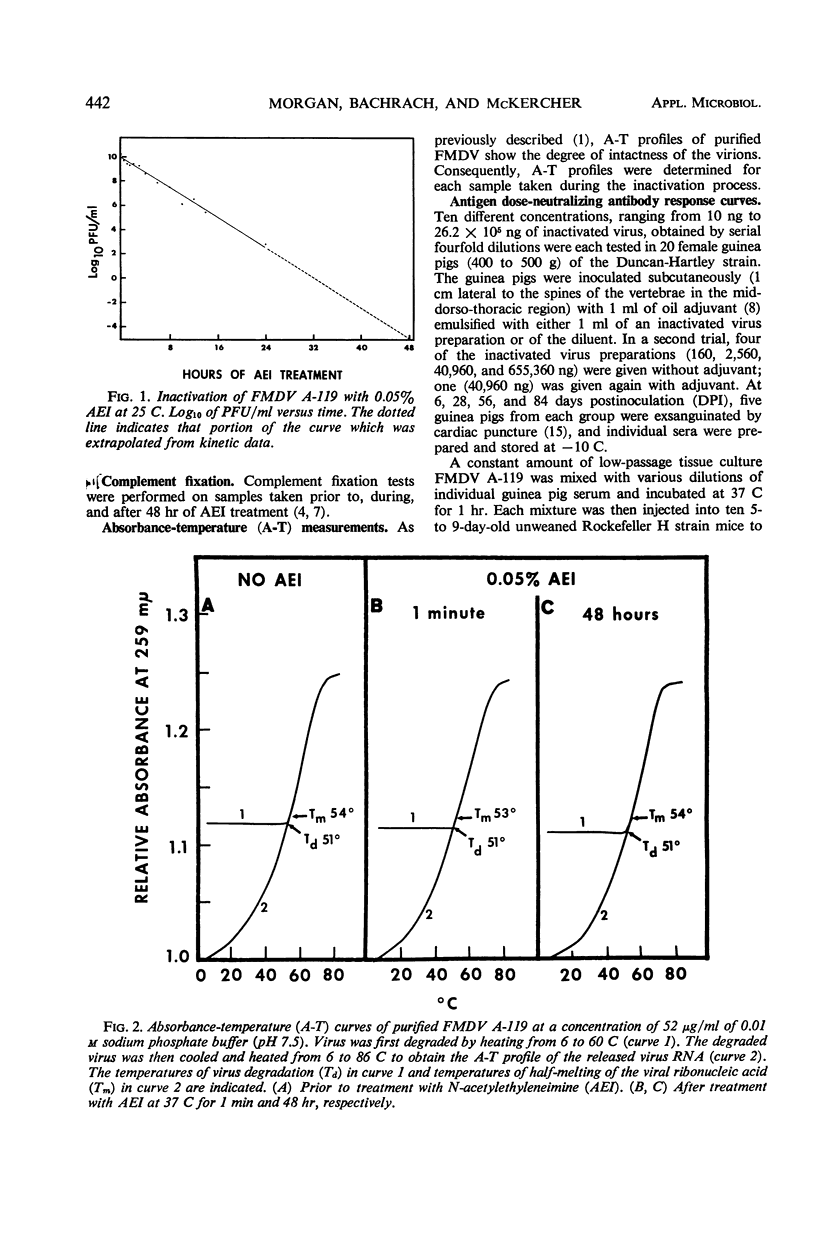
Quantitative antigen dose-neutralizing antibody response curves were established in guinea pigs for purified foot-and-mouth disease virus (FMDV), type A, strain 119, inactivated for 48 hr with N-acetylethyleneimine (AEI). Inactivation of FMDV by 0.05% AEI at 25 C occurred without virus degradation and followed first-order kinetics over a 108-fold decrease in plaque-forming units (PFU) extrapolating to 10-5 PFU/ml at 48 hr. The AEI-treated virus was administered in doses ranging from 10 ng to 2.62 mg, alone or emulsified in oil adjuvant. Sigmoidal dose-response curves were obtained with 160 ng as the minimum effective dose. The maximum effective dose was 163 μg and 2.62 mg or more at 6 and 28 through 84 days postinoculation, respectively. Oil adjuvant had little effect at 6 days postinoculation, but its use markedly increased the amount of neutralizing antibody obtained at the later testing periods.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHRACH H. L., CALLIS J. J., HESS W. R., PATTY R. E. A plaque assay for foot-and-mouth disease virus and kinetics of virus reproduction. Virology. 1957 Oct;4(2):224–236. doi: 10.1016/0042-6822(57)90060-0. [DOI] [PubMed] [Google Scholar]
- BACHRACH H. L. FOOT-AND-MOUTH DISEASE VIRUS: STRUCTURE AND MECHANISM OF DEGRADATION AS DEDUCED FROM ABSORBANCE-TEMPERATURE RELATIONSHIPS. J Mol Biol. 1964 Mar;8:348–358. doi: 10.1016/s0022-2836(64)80198-4. [DOI] [PubMed] [Google Scholar]
- BACHRACH H. L., TRAUTMAN R., BREESE S. S., Jr CHEMICAL PHYSICAL PROPERTIES OF VIRTUALLY PURE FOOT-AND-MOUTH DISEASE VIRUS. Am J Vet Res. 1964 Mar;25:333–342. [PubMed] [Google Scholar]
- BRADISH C. J., BROOKSBY J. B., TSUBAHARA H. The complement-fixation test in studies of components of the virus system of foot-and-mouth disease and its antibodies. J Gen Microbiol. 1960 Apr;22:392–404. doi: 10.1099/00221287-22-2-392. [DOI] [PubMed] [Google Scholar]
- BROWN F., HYSLOP N. S., CRICK J., MORROW A. W. THE USE OF ACETYLETHYLENEIMINE IN THE PRODUCTION OF INACTIVATED FOOT-AND-MOUTH DISEASE VACCINES. J Hyg (Lond) 1963 Sep;61:337–344. doi: 10.1017/s0022172400039620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWAN K. M., TRAUTMAN R. ANTIBODIES PRODUCED BY GUINEA PIGS INFECTED WITH FOOT-AND-MOUTH DISEASE VIRUS. J Immunol. 1965 Jun;94:858–867. [PubMed] [Google Scholar]
- Cowan K. M., Trautman R. Immunochemical studies of foot and mouth disease. I. Complement fixation reactions with isolated antigenic components. J Immunol. 1967 Oct;99(4):729–736. [PubMed] [Google Scholar]
- Cunliffe H. R., Graves J. H. Formalin-Treated Foot-and-Mouth Disease Virus: Comparison of Two Adjuvants in Cattle. Can J Comp Med Vet Sci. 1963 Aug;27(8):193–197. [PMC free article] [PubMed] [Google Scholar]
- GRAVES J. H., COWAN K. M., TRAUTMAN R. CHARACTERIZATION OF ANTIBODIES PRODUCED BY GUINEA PIGS INOCULATED WITH INACTIVATED FOOT-AND-MOUTH DISEASE ANTIGEN. J Immunol. 1964 Apr;92:501–506. [PubMed] [Google Scholar]
- GRAVES J. H., POPPENSIEK G. C. Determination of the optimal age range of mice for use in experimental studies with foot-and-mouth disease virus. Am J Vet Res. 1960 Jul;21:694–696. [PubMed] [Google Scholar]
- GRAVES J. H. TRANSFER OF NEUTRALIZING ANTIBODY BY COLOSTRUM TO CALVES BORN OF FOOT-AND-MOUTH DISEASE VACCINATED DAMS. J Immunol. 1963 Aug;91:251–256. [PubMed] [Google Scholar]
- Graves J. H., McKercher P. D., Farris H. E., Jr, Cowan K. M. Early response of cattle and swine to inactivated foot-and-mouth disease vaccine. Res Vet Sci. 1968 Jan;9(1):35–40. [PubMed] [Google Scholar]
- HENDERSON W. M. A comparison of different routes of inoculation of cattle for detection of the virus of foot-and-mouth disease. J Hyg (Lond) 1952 Jun;50(2):182–194. doi: 10.1017/s0022172400019537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYDE J. L. The use of solid carbon dioxide for producing short periods of anesthesia in guinea pigs. Am J Vet Res. 1962 May;23:684–685. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- McKercher P. D., Giordano A. R. Immune response of steers inoculated with chemically-treated foot-and-mouth disease virus preparations previously studied in swine. Arch Gesamte Virusforsch. 1967;20(2):190–197. doi: 10.1007/BF01241272. [DOI] [PubMed] [Google Scholar]
- POLATNICK J., BACHRACH H. L. PRODUCTION AND PURIFICATION OF MILLIGRAM AMOUNTS OF FOOT-AND-MOUTH DISEASE VIRUS FROM BABY HAMSTER KIDNEY CELL CULTURES. Appl Microbiol. 1964 Jul;12:368–373. doi: 10.1128/am.12.4.368-373.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. I. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN KINETICS AND ANTIGEN DOSE REQUIREMENT FOR INDUCTION. J Exp Med. 1964 Jan 1;119:1–19. doi: 10.1084/jem.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


