Figure 1.
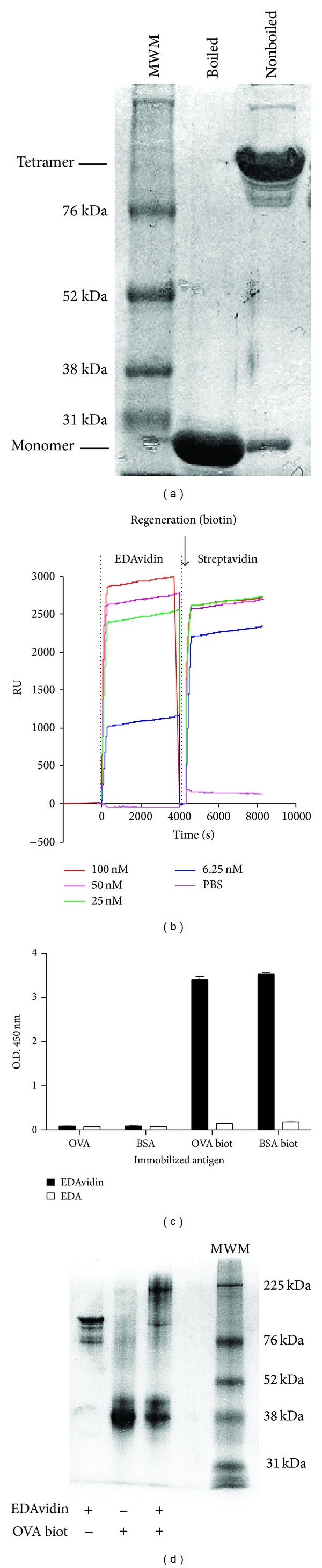
Recombinant EDAvidin tetramerizes and binds to biotinylated proteins. (a) SDS-PAGE of purified recombinant proteins stained with Coomassie blue (1: (MWM: molecular weight marker); 2: denatured EDAvidin; 3: nondenatured EDAvidin). (b) Surface plasmon resonance analysis of the capacity of EDAvidin and streptavidin to bind biotinylated proteins. Biotinylated ovalbumin was coated into the chip, and EDAvidin or streptavidin were injected at different concentrations. The surface of the chip was regenerated by the injection of an excess of 2 μM biotin before the injection of streptavidin (RU: surface plasmon resonance response units). (c) ELISA-based binding assays of EDAvidin to biotinylated proteins. Biotinylated or nonbiotinylated ovalbumin (OVA) and bovine serum albumin (BSA) were coated into the wells of ELISA plates. EDAvidin or EDA alone was added to the wells and after extensive washes, the plates were developed using rabbit polyclonal anti-EDA antibodies. (d) Binding assay of EDAvidin to biotinylated proteins by SDS-PAGEas 1: EDAvidin in its tetrameric form; 2: biotinylated OVA; 3: EDAvidin plus biotinylated OVA; 5: (MWM).
