Table 2. MDM2 and MDMX structure–activity relationships for triarylpyrroles 4c and 4m–z .
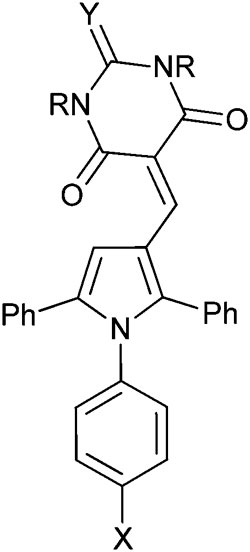
| ||||||
| Compound | R1 | R2 | X | Y | MDM2 IC50 (μM) a | MDMX IC50 (μM) a |
| 4c | H | H | Cl | S | 0.11 ± 0.03 | 4.2 ± 1.2 |
| 4m | H | H | Cl | O | 0.30 ± 0.03 | nd |
| 4n | H | H | Br | O | 0.18 ± 0.07 | nd |
| 4o | H | H | OMe | O | 1.9 ± 0.3 | 13 ± 7 |
| 4p | H | H | t-Bu | O | 1.9 ± 0.3 | nd |
| 4q | H | H | CN | O | 4.7 ± 1.9 | 7.0 ± 3.0 |
| 4r | H | H | CN | S | 0.20 ± 0.07 d | 0.90 ± 0.42 |
| 4s | H | H | NO2 | O | 0.15 ± 0.06 | 0.68 ± 0.18 |
| 4t | H | H | NO2 | S | 0.17 ± 0.09 d | 0.63 ± 0.12 |
| 4u | Et | Et | Cl | S | 0.30 ± 0.12 c | nd |
| 4v | Et | Et | Br | O | 0.89 ± 0.04 | 74% b |
| 4w | Et | Et | Br | S | 0.26 ± 0.05 | nd |
| 4x | Me | H | Cl | S | 0.11 ± 0.02 d | 28 ± 23 |
| 4y | Me | H | Br | O | 0.34 ± 0.08 | 35 ± 20 |
| 4z | Me | H | Br | S | 0.073 ± 0.002 | nd |
a n = 3, from resynthesised material.
b% inhibition at 50 μM.
c n = 6.
d n = 4; nd = not determined.
