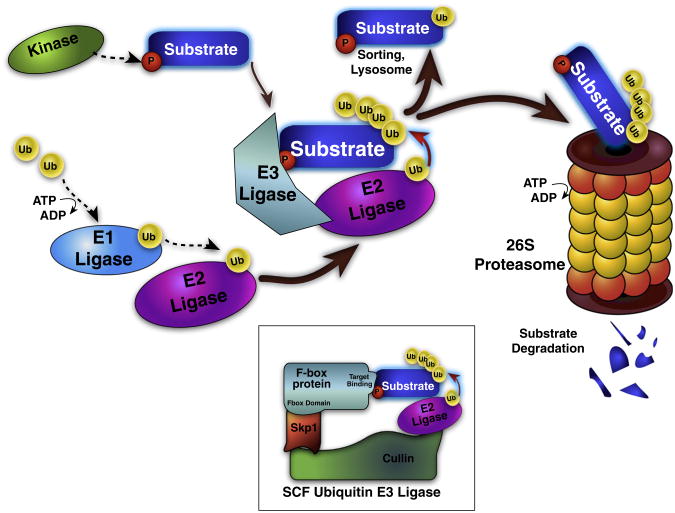
Fig. 1.
Schematic of the ubiquitin proteasome system and SCF E3 Ligase. Protein ubiquitination is a regulated, multi-step process. E1 ligases are loaded with ubiquitin (Ub) in an ATP-dependent fashion, and then transferred to E2 ligases. The same E2 ligase can bind many E3 ligases, which in turn can bind multiple target substrate proteins and coordinate Ub transfer from the E2 ligase to the substrate. E3 ligases bind specific substrate proteins based on substrate degron motifs usually consisting of a post-translational modification, such as phosphorylation. Ub addition tags the substrate for sorting, lysosomal destruction, or proteolytic cleavage and degradation by the 26S ubiquitin proteasome. Inset: the Skp1-cullin-F box (SCF) E3 Ub ligase represents the largest family of E3 ligase enzymes, with 68 identified human F-box proteins that interchangeably bind Skp1 and cullin; each F box selectively binds degron-containing substrate proteins, and mediates ubiquitination.