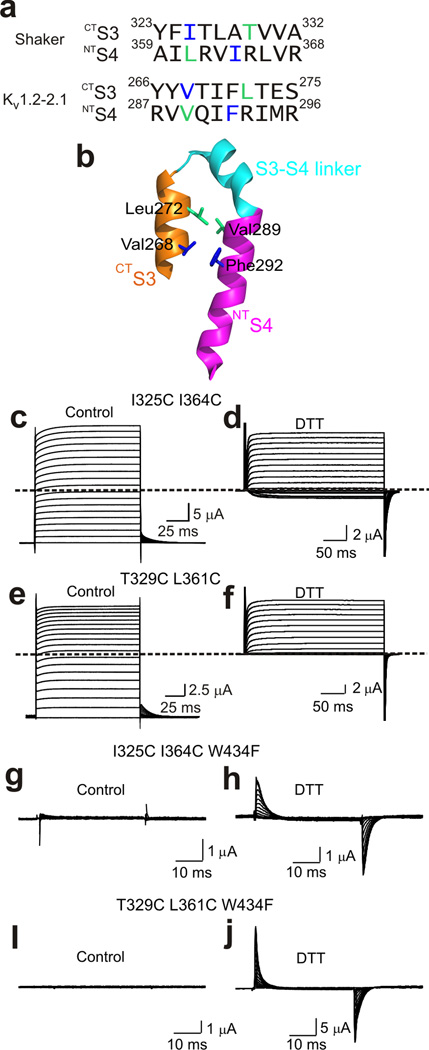
Figure 7.
Cysteine pairs between CTS3 and NTS4 that lock the channels in the open state. (a) Sequences of CTS3 and NTS4 of Shaker and Kv1.2–2.1 channels. The residue pairs in the Shaker sequence, whose substitution by cysteine lock the channel in the open state, are colored lime and blue. Corresponding residues in the Kv1.2–2.1 sequence are similarly colored. (b) Structure of Kv1.2–2.1’s CTS3 through NTS4 (PDB: 2R9R). Colored sticks correspond to the colored residues in the Kv1.2–2.1 sequence in a. (c–f) Ionic currents of the I325C I364C (c and d) or T329C L361C (e and f) double mutant without (control) or with exposure to 1 mM DTT (d, a few minutes; f, overnight). Currents were elicited by stepping membrane voltage from −100 mV (c and e) or −120 mV (d and f) to 100 mV (c and e) or 50 mV (d and f) in 10 mV increments. Traces shown in c and e were corrected for background currents obtained with 1 µM AgTx1 present. (g–j) Gating currents of channels containing the W434F mutation and the I325C I364C (g and h) or T329C L361C (i and j) double mutation without (control) or with exposure to 1 mM DTT (h, a few minutes; j, overnight). Currents elicited by stepping membrane voltage from −140 mV to 0 mV in 10 mV increments. Bathing solutions contained 100 mM K+ (c–f) or 5 mM K+ plus 95 mM Na+ (g–j).