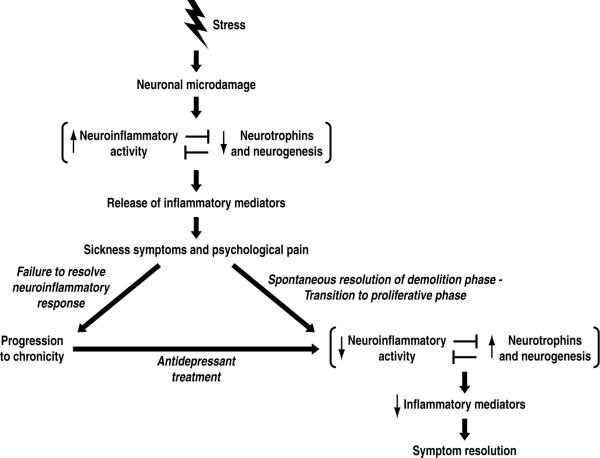
Figure 1.
Theoretical model for depression. In this model, a major adverse stressful life event (reviewed in section 2) leads to neuronal microdamage such as reduction of dendritic length, spines and branching in the hippocampus and prefrontal cortex (section 3). Such microdamage elicits a neuroinflammatory response (section 4), which inhibits neurogenesis and neurotrophin activity (section 3). The activated neuroinflammatory system releases inflammatory mediators which elicit sickness symptoms (section 5). In addition, these neuroinflammatory mediators hypersensitize psychological pain circuits by a similar mechanism to that by which they are known to hypersensitize physical pain circuits in the context of bodily injury (section 9.2). If the neuroinflammatory response fails to resolve, the depressive episode becomes chronic (section 10). On the other hand, a healthy inflammatory response will spontaneously resolve and transition to the proliferative phase of injury repair (section 9.1). In this transition, the decreased neuroinflammatory activity releases inhibition of neurotrophin activity and neurogenesis, and these trophic processes increase. As the injury repair nears completion, the release of proinflammatory mediators decrease, allowing depressive symptoms to remit. Because antidepressant treatments have anti-neuroinflammatory effects and lead to an increase in neurogenesis and neurotrophin expression (section 7), these treatments promote resolution of the injury repair response.