Abstract
Objective
Childhood and combat trauma have been observed to interact to influence amygdala volume in a sample of U.S. military veterans with and without PTSD. This interaction was assessed in a second, functionally-related fear system component, the pregenual and dorsal anterior cingulate cortex, using the same sample and modeling approach.
Method
Anterior cingulate cortical tissues (gray + white matter) were manually-delineated in 1.5 T MR images in 87 U.S. military veterans of the Vietnam and Persian Gulf wars. Hierarchical multiple regression modeling was used to assess associations between anterior cingulate volume and the following predictors, trauma prior to age 13, combat exposure, the interaction of early trauma and combat exposure, and PTSD diagnosis.
Results
As previously observed in the amygdala, unique variance in anterior cingulate cortical volume was associated with both the diagnosis of PTSD and with the interaction of childhood and combat trauma. The pattern of the latter interaction indicated that veterans with childhood trauma exhibited a significant inverse linear relationship between combat trauma and anterior cingulate volume while those without childhood trauma did not. Such associations were not observed in hippocampal or total cerebral tissue volumes.
Conclusions
In the dorsal anterior cingulate cortex, as in the amygdala, early trauma may confer excess sensitivity to later combat trauma.
Keywords: Posttraumatic stress disorder; Cingulate cortex; Stress, psychological; Combat disorders
Highlights
-
•
Childhood and combat trauma may interact to influence anterior cingulate cortex.
-
•
These findings partially replicate findings in amygdala.
-
•
Formally similar relations are found in endocrinological and psychometric data.
1. Introduction
Current evidence suggests large domains of the human brain exhibit dystrophic changes in response to early adversity. De Bellis et al. (1999, 2002) found total intracranial volume to be reduced in association with childhood maltreatment-related PTSD in two independent samples. Similar negative associations between early adversity and widely-distributed or whole-brain tissue volumes have been observed in healthy adult samples (Cohen et al., 2006; Dannlowski et al., 2012). Notwithstanding these generalized effects, available evidence suggests that medial frontal cortex is especially responsive to aggregated stressors. Cohen et al. (2006) observed associations between early adversity and reduced tissue volumes in sub- and pre-genual anterior cingulate cortex (ACC), while Dannlowski et al. (2012) observed a similar association involving neighboring ventromedial prefrontal cortex (see also van Harmelen et al., 2010). Ansell et al. (2012) observed an association between lifetime trauma (without regard to age) and lower gray matter density in a large cortical domain including sub-genual, pre-genual, and dorsal ACC. Though these studies have also reported associations between adversity and volumes of hippocampus (Dannlowski et al., 2012), insula (Ansell et al., 2012), and caudate (Cohen et al., 2006; Dannlowski et al., 2012), the literature considering impacts of stress on the brain independent of diagnosed posttraumatic stress disorder (PTSD) have consistently implicated ACC and adjacent medial prefrontal cortex.
Studies of aggregate stress on the brain and those of PTSD overlap in implicating the ACC. The ACC has been shown to be compromised in several studies of persons diagnosed with PTSD, though different subregions of this large structure have been highlighted in studies using different samples and methods (Kitayama et al., 2006; Rauch et al., 2003; Woodward et al., 2006b; Yamasue et al., 2003). There is now strong evidence that the ACC the amygdala together comprise a highly connected forebrain system devoted to managing responses to threat, and that impairment of the ACC's regulatory connectivity with the amygdala is a central feature of PTSD (Bremner et al., 1999; Etkin and Wager, 2007; Hamner et al., 1999; Shin and Liberzon, 2009).
In analyzing amygdala volume in this same sample of U.S. military veterans with and without combat-related PTSD, Kuo et al found evidence for multiple predictors (Kuo et al., 2012). PTSD was associated with a larger amygdala; however, once that association was accounted for, more combat exposure was associated with smaller amygdala volume. Moreover, this latter association interacted with early trauma such that only persons endorsing early trauma (i.e., prior to age 13) exhibited a negative association between combat trauma and amygdala volume. Given this result, the tight functional linkage between amygdala and ACC, and the apparent vulnerability of medial frontal cortex and ACC to stress, we asked whether the ACC might also evidence an interaction of early adversity and adult combat trauma. We followed this with analyses of Talairach-defined sectors of the ACC corresponding roughly to dorsal and ventral subregions in light of evidence associating them, respectively, with “cognitive” vs “emotional” (Bush et al., 2000), or “appraisal/expressive” vs “regulatory” (Etkin et al., 2011; Kalisch et al., 2006) involvement in negative affect. For purposes of comparison, we also considered whether this interaction was apparent in the hippocampus, another structure compromised in adult PTSD (Bremner et al., 1997; Karl et al., 2006; Woodward et al., 2006a) and/or total intracranial tissue volume, which is smaller in childhood maltreatment-related PTSD (De Bellis et al., 1999, 2002).
2. Methods
The study sample and imaging methodologies used here have been described in detail elsewhere (Woodward et al., 2006a,b). In brief, the sample included 87 military veterans who had served in the Vietnam Conflict or the Gulf War (GW). PTSD diagnosis and severity was assessed using the Clinician-Administered PTSD Scale (Blake et al., 1995). PTSD-positive (PTSD +) participants met criteria for current PTSD as a result of experiencing military trauma. PTSD-negative (PTSD −) participants also endorsed military trauma but were free of diagnosable PTSD, current or lifetime. Other Axis—I diagnoses were determined using the Structured Clinical Interview for the DSM-IV (First et al., 1995). Alcohol abuse/dependence-positive (ETOH +) subjects were classified based upon meeting lifetime, but not current alcohol abuse or dependence criteria. Lifetime trauma exposure was indexed using the Life Events Checklist (Gray et al., 2004) augmented by a structured follow-up interview establishing the A1 and A2 criteria and the period of trauma occurrence. Early traumatization was operationalized as any Criterion A event prior to age 13. Combat exposure was assessed using the Combat Exposure Scale (CES; Keane et al., 1989). GW veterans had a mean age of 38 years, and Vietnam veterans, 56 years. PTSD + participants had a mean age of 49.5 (8.6) years, and controls a mean age of 46.6 (10.5) years (t(85) = 1.39, n.s.). PTSD + participants scored lower on the vocabulary (53.6 (7.6) vs 47.7 (11.5); t(85) = 2.85, p = 0.005) subtest and much lower on the digit symbol substitution subtest (55.4 (15.1) vs 73.9 (12.2); t(85) = 3.08, p < 0.001) of the Wechsler Adult Intelligence Scale (WAIS, Psychological Corporation, 1997).
Magnetic resonance (MR) imaging was performed using 1.5 T General Electric Signa scanners (Milwaukee, WI) located at the Diagnostic Radiology Center of Veterans Affairs Palo Alto Health Care System and the Brain Imaging center of McLean Hospital (Belmont, Massachusetts). Images were acquired with a three-dimensional volumetric pulse sequence (repetition time [TR] = 35 ms, echo time [TE] = 6 ms, flip angle = 45°, number of excitations [NEX] = 1, matrix size = 256 × 192, field of view = 24 cm2, slice thickness = 1.5–1.7 mm, 124 slices). Skull-stripping, positional normalization, resampling to 0.933 cubic voxels, and tissue segmentation were performed in BrainImage (Reiss et al., 1998a). Manual delineation of the cingulate region by raters blind to participant identity and diagnosis followed a protocol developed by S.E. Cingulate gyri were traced in sagittal view on slices just lateral to the midline. Superior, inferior, lateral and medial boundaries of the cingulate cortex and white matter were traced in coronal view. Intra-class correlation coefficients over two independent raters indicated adequate inter-rater reliability for total cingulate volume measurements (ricc = 0.94). Excessive inter-subject variability in available landmarks mandated that subgenual cingulate be excluded from the delineation protocol. A dynamic Talairach grid was fitted to each brain yielding Talairach sectors B, C, D, E1 subdividing ACC (see Fig. 1, Woodward et al., 2006b). Sectors B and C were summed bilaterally to provide an estimate of ventral ACC and sector D used to estimate dorsal ACC. Hippocampal volume, summing gray plus white matter, was manually delineated as described in an earlier publication (Woodward et al., 2006a) by a different rater blind to subject identity and diagnosis who exhibited high intra-rater reliability. Intracranial volume was estimated as the sum of total cerebral tissue volume, summing gray and white matter, and sulcal and ventricular cerebrospinal fluid volumes. These values were obtained in a semi-automated fashion also using BrainImage (Reiss et al., 1998b). Both hippocampal and intracranial volumes were summed over hemispheres.
Hierarchical multiple linear regression modeling was used (SPSS 18). Predictors of total ACC volume (gray plus white, gray plus white) were entered in the order of presumed developmental impact: 1) presence/absence of early trauma, 2) CES score, 3) the interaction of early trauma and CES, and 4) PTSD diagnosis. This sequence of a base model plus three additional models each of additional candidate predictors are referred to below as models 1 through 4. All predictors were centered by subtraction of their medians (Kraemer and Blasey, 2004). Our preliminary hypothesis was that, as in the amygdala, early trauma and combat trauma would interact to influence ACC volume.
3. Results
It was preliminarily determined that there were no main effects of site (F(1,87) = 0.37, p = 0.55) nor of cohort (Vietnam vs Persian Gulf) (F(1,87) = 0.24, p = 0.63) on ACC volume, and that neither of these factors accounted for significant variance when added to the multiple linear regression in advance of the variables of interest. Similarly, entering age at step 1 did not account for significant variance in ACC volume (Fchange(1,85) = 0.042, p = 0.84). Furthermore, none of these variables, or their combinations, when added at step 1, substantially impacted the beta weights comprising the final models.
When entered first, childhood trauma alone did not account for significant variance in ACC volume (β = − 0.118, t = − 1.67, n.s.). The addition of combat trauma as indexed by the CES score (model 2) did not account for significant additional variance in ACC volume variance (Fchange(1,84) = 3.43, p < 0.10). Neither childhood trauma nor CES score alone accounted for unique variance in ACC volume (childhood trauma: β = − 0.15, t = − 1.42, n.s.; CES: β = − 0.20, t = − 1.85, n.s.); however, the simultaneous regression model was nominally significant (adjusted R2 = 0.05, F(2,84) = 3.2, p < 0.048). With the addition of the interaction of childhood trauma and CES, model 3 accounted for significant additional variance in ACC volume (Fchange(1,83) = 5.59, p < 0.05) with the interaction, itself, accounting for significant unique variance (β = − 0.25, t = − 2.36, p < 0.05). The addition of PTSD diagnosis (model 4) also accounted for additional variance in ACC volume (Fchange(1,82) = 10.90, p < 0.001). In model 4, the interaction of childhood and combat trauma continued to explain unique variance in ACC volume (β = − 0.26, t = − 2.68, p < 0.009). Model 4 accounted for 19% of ACC volume (F(4,82) = 6.15, p < 0.001).
The interaction of childhood and combat trauma is depicted in Fig. 1, where it can be seen that among combat veterans denying trauma prior to age 13, CES score and ACC volume were uncorrelated. In contrast, among those endorsing trauma prior to age 13, CES score and ACC volume were negatively correlated (r(37) = − 0.41, p < 0.01).
Fig. 1.
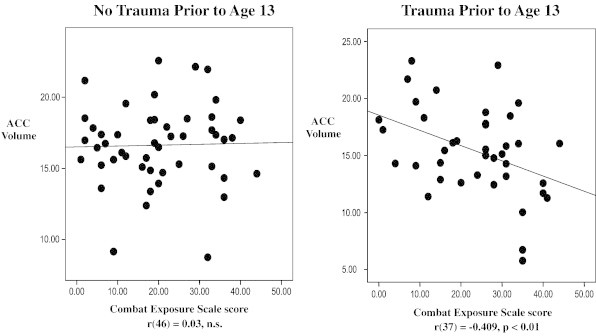
Illustration of the early trauma by combat exposure interaction on ACC volume.
We also tested for variation in model fit in separate estimates of ventral and dorsal ACC volume. In ventral ACC, neither models 1 nor 2 were associated with significant increases in variance explained; however, models 3 and 4 did yield significant increases in variance explained (model 3: Fchange(1,83) = 5.61, p = 0.02; model 4: Fchange(1,82) = 10.23, p = 0.002), with the interaction of childhood and combat trauma again explaining significant unique variance after the entry of the presence/absence of a PTSD diagnosis (β = − 0.27, t = − 2.66, p < 0.009). In contrast, in dorsal ACC, both models 1 and 2 were associated with significant increase in variance explained (model 1: Fchange(1,85) = 8.51, p = 0.005; model 2: Fchange(1,84) = 4.09, p = 0.046). Moreover, childhood trauma accounted for unique variance in dorsal ACC volume in models 1, 2 and 3 (model 1: β = − 0.30, t = − 2.92, p = 0.005; model 2: β = − 0.27, t = − 2.66, p = 0.009; model 3: β = − 0.25, t = − 2.40, p = 0.019); and combat trauma accounted for unique variance in dorsal ACC in models 2 and 3 (model 2: β = − 0.21, t = − 2.02, p = 0.048; model 3: β = − 0.23, t = − 2.21, p = 0.03). However, with regard to dorsal ACC volume, adding the interaction of childhood and combat trauma (model 3) was not associated with an increase in variance explained (Fchange(1,83) = 2.53, n.s.); and though the omnibus model 4 explained significant variance in dorsal ACC volume (F(4,86) = 5.23, p < 0.001), PTSD was the only significant predictor in that model (β = − 0.25, t = − 2.12, p = 0.034). In summary, dorsal ACC volume appeared to be associated with childhood and adult trauma, while ventral ACC volume appeared to be associated with both the interaction of childhood and adult trauma and with PTSD.
Similar analyses were repeated for left and right ACC volumes. In the left ACC, childhood trauma was a significant predictor in models 1 and 2 (model 1: β = − 0.22, t = − 2.11, p < 0.05; model 2: β = − 0.22, t = − 2.08, p < 0.05). Thereafter, only a diagnosis of PTSD (in model 4) was associated with left ACC volume (β = − 0.45, t = − 3.92, p < 0.001). In the right hemisphere, combat exposure was associated with smaller ACC volume in models 2, 3 and 4 (model 2: β = − 0.27, t = − 2.58, p < 0.05: model 3: β = − 0.30, t = − 2.86, p < 0.01; model 4: β = − 0.4, t = − 2.06, p < 0.05). Similarly, the interaction of early trauma and CES was associated with smaller right ACC volume in models 3 and 4 (model 3: β = − 0.23, t = − 2.16, p < 0.05; model 4: β = − 0.233, t = − 2.24, p < 0.05). PTSD diagnosis was not associated with significant variance in right ACC in model 4 (β = − 0.16, t = − 1.35, n.s.). In summary, the interaction of combat exposure with early trauma accounted for variance in right but not left ACC volume.
Finally, we addressed whether the same predictors could similarly model variance in hippocampus and/or total cerebral tissue volume. As summarized in Table 1, neither of these candidate volumes exhibited simultaneous associations with PTSD diagnosis and the interaction of early and combat trauma.
Table 1.
Standardized beta weights for final model (model 4) for ACC, hippocampal (left + right, gray + white) and cerebral tissue volumes (left + right, gray + white) regressed on early trauma, combat exposure, the interaction of early trauma and combat exposure, and PTSD diagnosis.
| Brain region | Early trauma | Combat exposure | Early trauma × combat exposure | PTSD diagnosis |
|---|---|---|---|---|
| ACC | − 0.01 | − 0.08 | − 0.26⁎⁎ | − 0.37⁎⁎⁎ |
| Hippocampus | − 0.12 | 0.08 | 0.14 | − 0.25⁎ |
| Cerebral tissue | − 0.11 | 0.16 | 0.07 | − 0.17 |
p < 0.05.
p < 0.01.
p < 0.001.
4. Discussion
Kuo et al found that early trauma interacted with combat exposure such that veterans endorsing childhood trauma exhibited a negative relationship between combat exposure severity and amygdala volume while those denying childhood trauma did not. Here, we have observed formally similar associations in the anatomically distinct but functionally related ACC. These results provide indirect support for the observations of Kuo et al. (2012). Though cross-sectional in nature, they converge with results from several domains demonstrating that developmental adversity and combat trauma can interact to influence risk for PTSD (Cabrera et al., 2007; LeardMann et al., 2010; McLaughlin et al., 2010). These associations were not observed in the volumes of two comparison structures implicated in adult and pediatric PTSD, the hippocampus and total cerebrum, suggesting specificity to the amygdala and ACC.
The functional relationship between ACC and amygdala has emerged as a central focus of investigation of the neurobiology of PTSD (Bremner et al., 2008; Liberzon and Martis, 2006; Shin and Liberzon, 2009). The current results suggest that early trauma might influence both amygdala and ACC in parallel to confer sensitivity to combat trauma. This parallelism could reflect their shared status as core components of the fear system and/or more directly manifest a consequence of their mechanistic interactions. The observed pattern is simpler in ACC than in amygdala, as both PTSD and the interaction of early trauma and combat exposure were associated with smaller ACC volume. One implication which may be extracted from these findings is that if early and/or aggregate stressors predispose persons to PTSD, and if structural modifications of these regions are associated with both those stressors and with PTSD, then statistical associations between medial frontal compromise and adversity will generally be attenuated in the presence of PTSD. These arguments lead to the conclusion that assessments of both early and adult trauma may be necessary to provide a full accounting of associations between medial frontal cortex and PTSD.
Within ACC, the current results suggest that the dorsal subdivision may be preferentially associated with simple additive effects of trauma exposure while the ventral subdivision is associated with “downstream” effects, the interaction of childhood trauma and combat exposure and PTSD. These apparently divergent associations provide support for the parcellation of cingulate contributions to PTSD. They also provide support the separability, in the brain, of trauma and PTSD, in conformance with the conventional framework based on the epidemiology of the disorder (Bryant et al., 2010). Comparison of associations with left versus right ACC provides further support for parcellation as the former but not the latter was, here, associated with the interaction of childhood and combat trauma. The search for additional simple or interactive predictors of structural compromise in medial frontal subregions could aid the development of a comprehensive explanation of their contributions to PTSD and related disorders. As noted in the Introduction, structural studies of the ACC in PTSD have failed to converge on a single subregion. Notwithstanding the current findings, among studies reporting structural compromise in ACC in PTSD only Chen et al. (2006) has reported a laterality effect. Analogously, if we consider prominent functional parcellations of dorsal versus ventral ACC (e.g. “cognitive” versus “emotional” or “appraisal/expressive” versus “regulatory”), it is difficult to exclude any of these nominal domains as irrelevant to PTSD psychopathology.
An important limitation of this study is the absence of data regarding subgenual ACC. A number of studies have implicated this most ventral subregion of the ACC in PTSD (Etkin and Wager, 2007; Hayes et al., 2012) while others have described its extensive interconnections with the amygdala (Gutman et al., 2009; Johansen-Berg et al., 2008). It is therefore unfortunate that anatomic variability sometimes presents a challenge to volumetric analysis of this structure (Fornito et al., 2006; Fornito et al., 2008; Spasojevic et al., 2011). A further limitation is the high comorbidity of PTSD with Major Depressive Disorder (78%) precluding their discrimination as predictors of ACC volume. Additional limitations are the absence of an objective measure of childhood trauma and of a measure of socioeconomic status.
Acknowledgments
Funding for this work was supported under a VA/DoD assistance agreement to Drs. Woodward and Kaloupek from the U.S. Army Medical Research and Material Command (DoD) administered through the Institute for Medical Research (VA). Drs. Eliez and Schaer were supported by the National Center of Competence in Research (NCCR) “SYNAPSY — The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (51AU40_125759). We thank the Research Services of the VA Palo Alto and VA Boston Healthcare Systems for their assistance. Important institutional and technical support was also provided by the following individuals: Carla Ambriz, Steven Blank, Thomas J. Brosnan, Eileen Connolly, Michael A. Dove, John E. Drace, Blaise Frederick, Gary E. Gold, Frederick Kanter, Matthew O. Kimble, Catherine J. Kutter, Lorraine P. Leskin, Anil Patwardhan, Rebecca S. Prestel, Susan P. Proctor, Allan L. Reiss, Chris C. Streeter, Perry F. Renshaw, Eric Schmitt, Ann Smith, Patricia Spezia, Wendy K. Stegman, Erica Stone, Kelly Teresi and Rosemond Villafuerte.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Ansell E.B., Rando K., Tuit K., Guarnaccia J., Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Randall P., Vermetten E., Staib L., Bronen R.A., Mazure C., Capelli S., McCarthy G., Innis R.B., Charney D.S. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biological Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Staib L.H., Kaloupek D., Southwick S.M., Soufer R., Charney D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Elzinga B., Schmahl C., Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Progress in Brain Research. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., Silove D. The psychiatric sequelae of traumatic injury. The American Journal of Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabrera O.A., Hoge C.W., Bliese P.D., Castro C.A., Messer S.C. Childhood adversity and combat as predictors of depression and post-traumatic stress in deployed troops. American Journal of Preventive Medicine. 2007;33:77–82. doi: 10.1016/j.amepre.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Chen S., Xia W., Li L., Liu J., He Z., Zhang Z., Yan L., Zhang J., Hu D. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Research. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Cohen R.A., Grieve S., Hoth K.F., Paul R.H., Sweet L., Tate D., Gunstad J., Stroud L., McCaffery J., Hitsman B., Niaura R., Clark C.R., McFarlane A., Bryant R., Gordon E., Williams L.M. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Clark D.B., Casey B.J., Giedd J.N., Boring A.M., Frustaci K., Ryan N.D. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Shifflett H., Iyengar S., Beers S.R., Hall J., Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Science. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research Department, NY State Psychiatric Institute; New York: 1995. Structured Clinical Interview for Axis I DSM-IV Disorders — Patient Edition (SCID-I/P) [Google Scholar]
- Fornito A., Whittle S., Wood S.J., Velakoulis D., Pantelis C., Yucel M. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. NeuroImage. 2006;33:843–854. doi: 10.1016/j.neuroimage.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Fornito A., Wood S.J., Whittle S., Fuller J., Adamson C., Saling M.M., Velakoulis D., Pantelis C., Yucel M. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Human Brain Mapping. 2008;29:222–236. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.J., Litz B.T., Hsu J.L., Lombardo T.W. Psychometric properties of the life events checklist. Assessment. 2004;11:330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- Gutman D.A., Holtzheimer P.E., Behrens T.E., Johansen-Berg H., Mayberg H.S. A tractography analysis of two deep brain stimulation white matter targets for depression. Biological Psychiatry. 2009;65:276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner M.B., Lorberbaum J.P., George M.S. Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depression and Anxiety. 1999;9:1–14. [PubMed] [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H., Gutman D.A., Behrens T.E., Matthews P.M., Rushworth M.F., Katz E., Lozano A.M., Mayberg H.S. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R., Wiech K., Critchley H.D., Dolan R.J. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. NeuroImage. 2006;30:1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Karl A., Schaefer M., Malta L.S., Dorfel D., Rohleder N., Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Keane T.M., Fairbank J.A., Caddell J.M., Zimering R.T., Taylor K.L., Mora C.A. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment. 1989;1:53–55. [Google Scholar]
- Kitayama N., Quinn S., Bremner J.D. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. Journal of Affective Disorders. 2006;90:171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H.C., Blasey C.M. Centring in regression analyses: a strategy to prevent errors in statistical inference. International Journal of Methods in Psychiatric Research. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J.R., Kaloupek D.G., Woodward S.H. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Archives of General Psychiatry. 2012;69:1080–1086. doi: 10.1001/archgenpsychiatry.2012.73. [DOI] [PubMed] [Google Scholar]
- LeardMann C.A., Smith B., Ryan M.A. Do adverse childhood experiences increase the risk of postdeployment posttraumatic stress disorder in US Marines? BMC Public Health. 2010;10:437. doi: 10.1186/1471-2458-10-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Martis B. Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Conron K.J., Koenen K.C., Gilman S.E. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation . Third Revision. Harcourt Brace; San Antonio: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- Rauch S.L., Shin L.M., Segal E., Pitman R.K., Carson M.A., McMullin K., Whalen P.J., Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Reiss A.L., Hennessey J.G., Rubin M., Beach L., Abrams M.T., Warsofsky I.S., Liu A.M., Links J.M. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. Journal of Computer Assisted Tomography. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Reiss A.L., Hennessey J.G., Rubin M., Beach L., Abrams M.T., Warsofsky I.S., Liu A.M., Links J.M. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. Journal of Computer Assisted Tomography. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. Neuropsychopharmacology; 2009. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojevic G.D., Malobabic S., Suscevic D., Stijak L., Nikolic V., Gojkovic I. Morphological variability of the subcallosal area of man. Surgical and Radiologic Anatomy. 2011;33:313–318. doi: 10.1007/s00276-010-0689-2. [DOI] [PubMed] [Google Scholar]
- van Harmelen A.L., van Tol M.J., van der Wee N.J., Veltman D.J., Aleman A., Spinhoven P., van Buchem M.A., Zitman F.G., Penninx B.W., Elzinga B.M. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Woodward S.H., Kaloupek D.G., Streeter C.C., Kimble M.O., Reiss A.L., Eliez S., Wald L.L., Renshaw P.F., Frederick B.B., Lane B., Sheikh J.I., Stegman W.K., Kutter C.J., Stewart L.P., Prestel R.S., Arsenault N.J. Hippocampal volume, PTSD, and alcoholism in combat veterans. The American Journal of Psychiatry. 2006;163:674–681. doi: 10.1176/ajp.2006.163.4.674. [DOI] [PubMed] [Google Scholar]
- Woodward S.H., Kaloupek D.G., Streeter C.C., Martinez C., Schaer M., Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biological Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Yamasue H., Kasai K., Iwanami A., Ohtani T., Yamada H., Abe O., Kuroki N., Fukuda R., Tochigi M., Furukawa S., Sadamatsu M., Sasaki T., Aoki S., Ohtomo K., Asukai N., Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]


