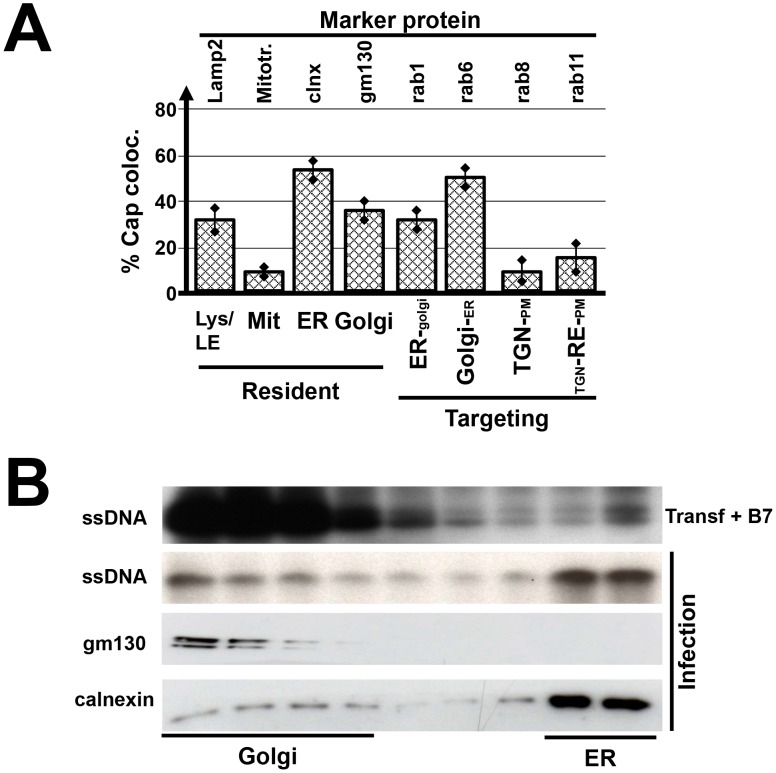
Figure 2. Colocalization of MVM virions with cytoplasmic marker proteins.
A9 cells were infected with MVMp (30 pfu/cells) and analyzed 24 h p.i. for the presence of newly synthesized progeny virions. (A) Cells grown on spot slides were fixed with paraformaldehyde and analyzed by confocal laser scanning microscopy after double-staining using specific antisera for the indicated cell proteins and MVM capsids (Fig. S1). Virus neutralizing antibodies were added after the initial infection to prevent cell re-infections with progeny viruses. Colocalization was quantified by Image J analyzing infected cells from three individual experiments with >10 cells. Lys/LE, lysosomes/late endosomes (lamp2); Mit, mitochondria (mitotracker); ER, endoplasmic reticulum (calnexin [clnx]); TGN, trans golgi network; RE, recycling endosomes; PM, plasma membrane. (B) Cellular extracts were fractionated to separate golgi- versus ER-membrane structures by nycodenz gradient centrifugation. Progeny virions were detected by southern blotting measuring single-stranded virion DNA, the presence of gm130 (golgi) and calnexin (ER), respectively, by western blotting. To preclude interference from input viruses, a control was made by transfecting cells with MVM infectious DNA clone. Transfected cells were further incubated in the presence of neutralizing B7 antibodies to ensure that infection is limited to a single round and cell do not get reinfected with progeny viruses (Transf+B7 panel).