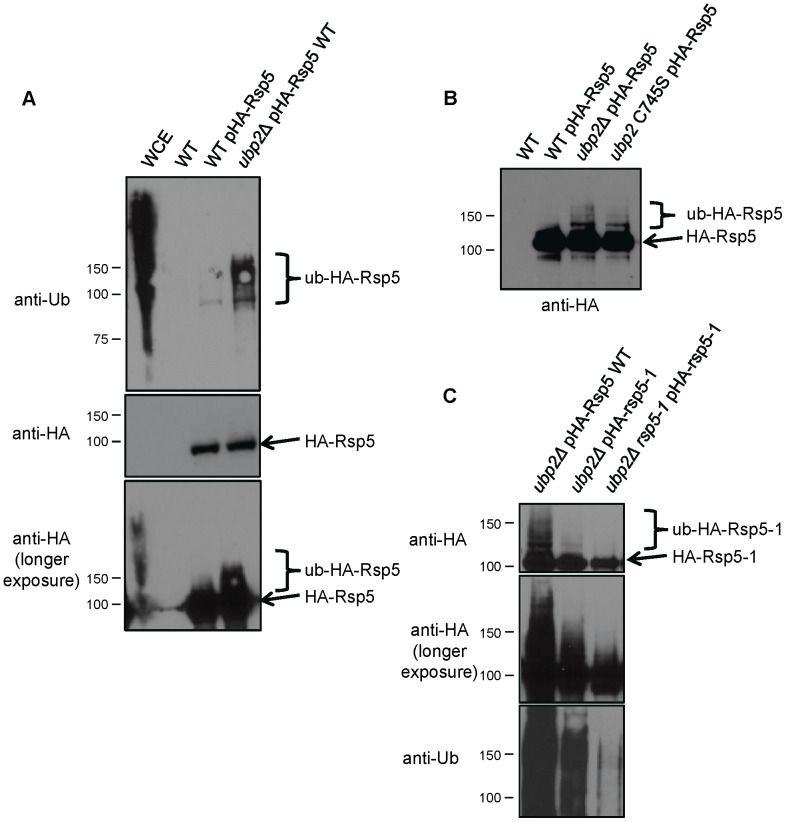
Figure 1. Rsp5 is stably ubiquitinated in the absence of Ubp2.
(A) Western blot showing ubiquitinated and unmodified forms of Rsp5 in vivo. A plasmid expressing epitope-tagged Rsp5 (pHA-Rsp5) was transformed into wildtype and ubp2Δ cells. Cells were lysed in native buffer, and Rsp5 immunoprecipitated with anti-HA antibodies. After transfer to nitrocellulose, Rsp5 species were visualized using anti-ubiquitin (top panel) and anti-HA antibodies (middle; longer exposure at bottom). Whole cell extract from cells lacking plasmid (WCE) and immunoprecipitates from these same cells (WT) were run as positive and negative controls, respectively. (B) Experiment performed as in (A), except with an increased exposure time in order to visualize lower mobility Ub-HA-Rsp5 species. Accumulation of ubiquitinated HA-Rsp5 is evident in a strain bearing a catalytically inactive mutant allele in UBP2 (ubp2 C745S). (C) Rsp5 is auto-ubiquitinated. Plasmids expressing either wildtype Rsp5 (pHA-Rsp5 WT) or a conditional catalytic mutant of Rsp5 (pHA-rsp5-1) were transformed into haploid yeast lacking UBP2 alone or both UBP2 and a fully functional copy of RSP5 (rsp5-1). Prior to analysis, the strains were grown overnight, diluted in fresh media, and incubated at the non-permissive temperature (37°C) to inactivate Rsp5. Cell lysates were immunoprecipitated with anti-HA antibodies, and the blot first probed with anti-ubiquitin, followed by extended wash steps to rinse off residual antibody, and finally Rsp5 was detected with anti-HA antibodies. A darker exposure of the anti-HA blot is shown to highlight the lack of low-mobility (multi/poly-ubiquitinated) forms of Rsp5-1.