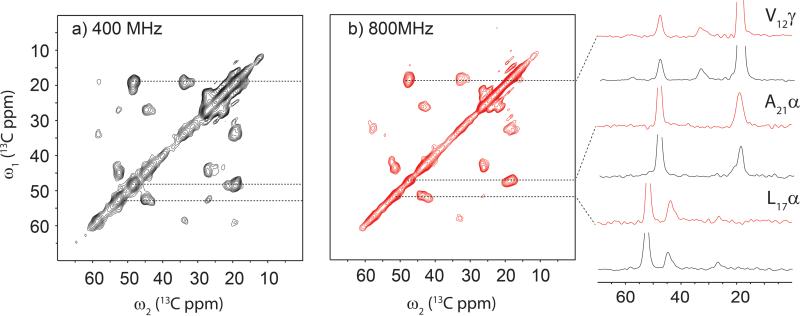
Figure 6.
A comparison of 2D 13C/13C correlation SSNMR spectra of Cu2+-bound amyloid fibrils of Aβ(1-40) obtained at 1H NMR frequencies of (a) 400 MHz with traditional scheme and at (b) 800 MHz with PACC scheme, together with (c) 1D slices. The total experimental times were (a) 32 h and (b) 1.9 h for (a) 2 mg and (b) 1 mg of the Aβ sample that was uniformly 13C- and 15N-labeled at selected residues Phe-4, Gly-9, Val-12, Leu-17, and Ala-21. The data in (a, b) were processed with Gaussian broadening of 1.0 ppm in both t1 and t2 periods. The data in (a) were acquired with the t1 and t2 periods of 4 ms and 8 ms, respectively on a Bruker Avance III 400 MHz spectrometer equipped with a homebuilt 2.5-mm CPMAS triple-resonance probe. For (a), a standard 2D 13C/13C correlation sequence was used under MAS at 20 kHz with high-power 1H TPPM decoupling at the RF intensity of 90 kHz.45 During the mixing period of 1.6 ms, the fpRFDR sequence45 was applied with 13C π-pulses of 15-μs width. The data in (b) was acquired in the PACC scheme with the t1 and t2 periods of 2 ms and 8 ms, respectively on a Bruker Avance 800 MHz spectrometer equipped with a JEOL 1-mm double-resonance CPMAS probe. In (b), 1H lpTPPM decoupling was applied at 12.5 kHz in the t1 and t2 periods, and the fpRFDR mixing was applied during the mixing period of 1.92 ms with 13C π-pulses of 7 μs widths.