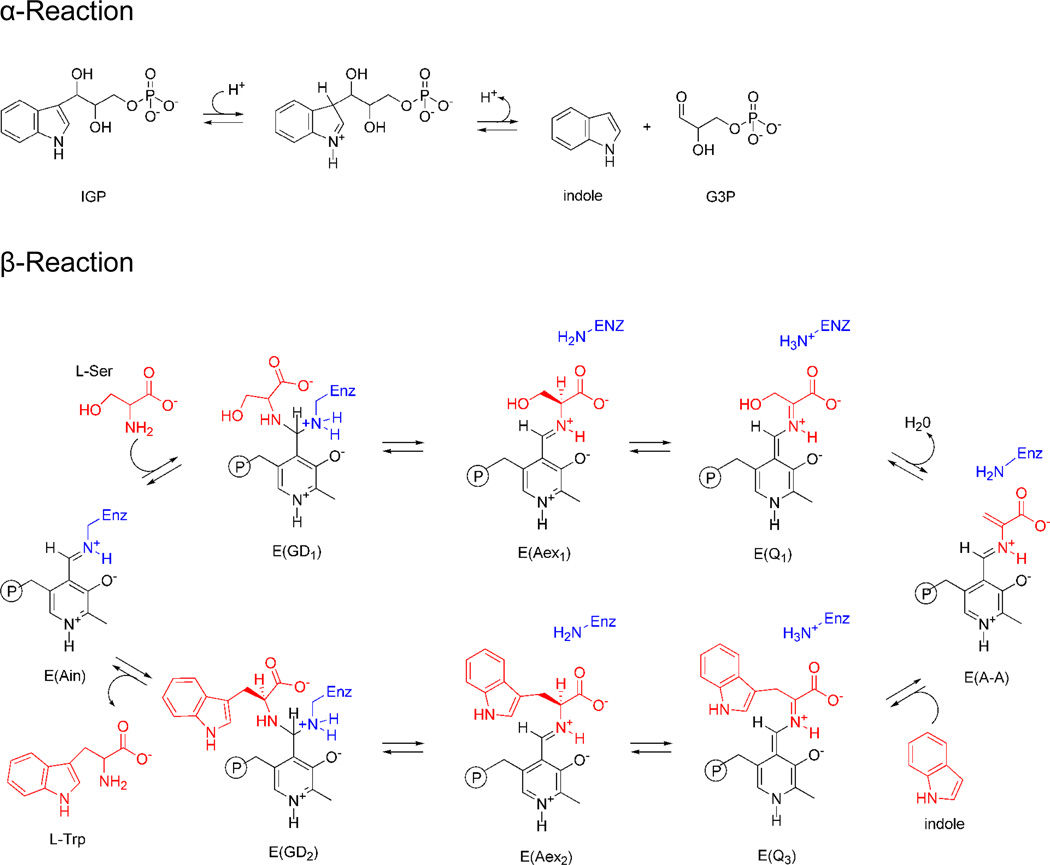
Scheme I.
Mechanistic pathway for the biosynthesis of L-Trp in tryptophan synthase. In the α-site, 3-indole-D-glycerol-3′-phosphate (IGP) is cleaved to D-glyceraldehyde-3-phosphate (G3P) and indole. In stage I of the β-reaction, L-Ser reacts with the internal aldimine, E(Ain), giving in sequence gem-diamine, E(GD1), L-Ser external aldimine, E(Aex1), quinonoid, E(Q1), and aminoacrylate Schiff base, E(A-A), species and a water molecule. In stage II, indole, channeled from the α-site, makes a nucleophilic attack on E(A-A) giving E(Q3), E(Aex2), E(GD2) intermediates and finally the product, L-Trp.