Abstract
Altered production of cytokines can result in pathologies ranging from autoimmune diseases to malignancies. The Janus Kinases family is a small group of receptor-associated signaling molecules that is essential to the signal cascade originating from type I and type II cytokine receptors. Inhibition of tyrosine kinases enzymatic activity using small molecules has recently become a powerful tool for treatment of several malignancies. Twenty years after the discovery of these enzymes, two inhibitors for this class of kinases have been approved for clinical use and others are currently in the final stage of development. Here we review the principles of cytokines signaling, we summarize our current knowledge of the approved inhibitors, and briefly introduce some of the inhibitors that are currently under development.
Introduction
Autoimmune diseases, allergies, and even malignancies are often the consequence of a persistent imbalance within complex immune mechanisms. The actions of several cytokines are the basis of these complex processes, as these soluble factors play a critical role in the control of the immune responses and inflammatory processes (1). Furthermore, several human genome-wide expression studies have linked various cytokines, and their receptors or molecules involved in their signaling cascades to immune-mediated and inflammatory diseases (2). Not surprisingly then, modulation of cytokine functions has been the focus of intensive research and drug development. In fact, drugs targeting cytokines or their receptors have become the main weapon in the armamentarium of physicians dealing with, for example, autoimmune diseases.
Better knowledge of the events occurring upon cytokines binding to their specific receptors resulted in a lot of interest in the possibility to target these intracellular signaling cascades. The Janus Kinase (JAK)-Signal Transducers and Activator of Transcription (STAT) pathway was discovered about 20 years ago (3) and this linear cascade mediates signaling between surface receptors and cellular responses. The four JAKs (JAK1, 2, 3 and TYK2) have been shown to be critical components of cytokine-mediated effects.
Here, we summarize the biology of JAKs-mediated signals in the context of the immune response. We will also review the drugs developed so far to inhibit JAKs. Finally we will discuss the drugs already available to physicians, as well as those under development, and how this new class of small molecules could impact the treatment of immune-mediated and other disorders.
Cytokine receptor signaling: the JAK-STAT cascade
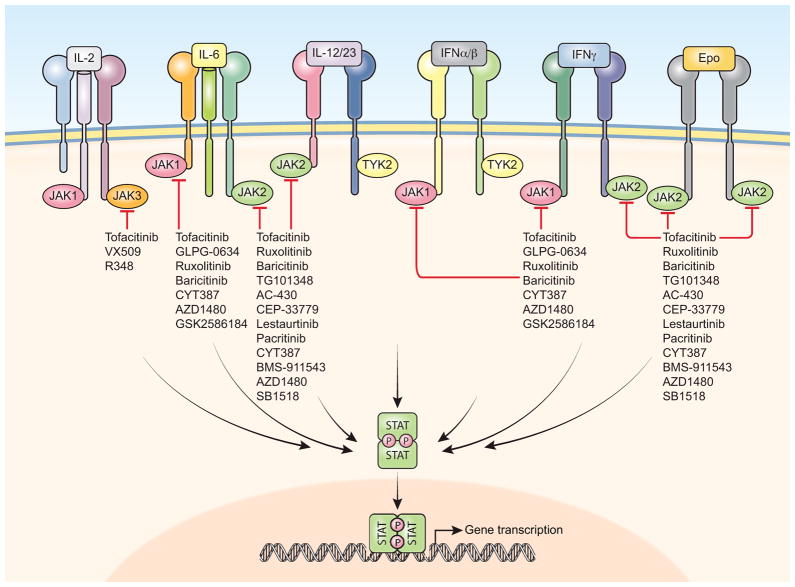
Soluble cytokines (and some growth factors) bind to a structurally distinct class of integral membrane receptors known as Type I and Type II cytokine receptors (1). The intracellular portions of these receptors do not have intrinsic enzymatic activity but possess structural features that allow the recruitment of a variety of signaling molecules. Among these, the JAKs are a subgroup of non-receptor tyrosine kinases that transduce signals specifically from cytokine receptors, and whose enzymatic activity is essential for the biological activity of cytokines. Upon ligand binding, JAKs are phosphorylated on specific tyrosine and serine residues, and become enzymatically active. The kinase activity of JAKs is directed towards the JAKs themselves, the intracellular portion of the receptor, and several other substrates including the members of the STAT family of transcription factors. STATs (STAT1 though STAT6) have specific and distinct effects on gene transcription in numerous cell types, including immune cells, and are critical in processes such as cell proliferation and differentiation. Upon phosphorylation by the JAKs, STATs dimerize and translocate to the nucleus where they bind DNA, and in turn, regulate gene expression (Figure 1).
Figure 1. JAK inhibitors prevent JAK activation.
The signaling cascade that originates upon binding of the cytokines to their specific receptors is blunted by the action of specific JAK inhibitors. JAKs are no longer capable to phosphorylate substrates like STATs and, therefore, cytokine-dependent gene regulation is prevented.
Cytokines and growth factors act on various organs and, accordingly, JAK proteins are expressed in all the cell types. JAK3 is the only exception, since it’s predominantly expressed in hematopoietic cell lineages (4). The structure of the JAK has been covered extensively before (5). Briefly, the kinase domain is located on the C-terminus of the molecule and is preceded by a pseudokinase domain, which is structurally similar, and, in JAK2, has been shown to phosphorylate two negative regulatory sites and therefore serving an important regulatory role (6). The relative importance of the pseudokinase domain has become apparent when mutations in this domain in JAK2 have been shown to be the cause of various hematologic disorders (7). Next to the pseudokinase domain is a Src Homology 2 (SH2) domain, which could be indicative of a capacity to recognize and bind phosphorylated tyrosine residues (although this has not yet been proven). The N-terminus encodes a FERM (4.1 protein, Ezrin, Radixin, Moesin) domain which allows JAKs to interact with the intracellular portion of the receptors and which also has a role in controlling the enzymatic activity (8).
The importance of the JAKs for many biological processes was evidenced by the generation of animals lacking their expression. Both JAK1 and JAK2 nullizygous genotypes are embryonically lethal in mice and cause major developmental defects including deficiencies in lymphopoiesis and erythropoiesis (9) JAK1 mutations in humans have not been reported. Conversely, JAK2 protein alterations have been associated with several diseases. It was found that JAK2 could fuse to a part of the transcription factor TEL to generate a fusion protein responsible for Sézary syndrome, a type of cutaneous lymphoma (10). Other fusion proteins have been reported with PCM1 and Sec31A as cause of leukemias and lymphomas (11, 12). Moreover JAK2 mutations were found to be the cause underlying around 95% of polycythemia vera (PV) patients and approximately 50% of essential thrombocythemia (ET) and myelofibrosis (hereby we will collectively refer to this diseases as myeloproliferative diseases (MPD)) (13). The most common mutation associated with these diseases is the V617F mutation in the pseudokinase domain, which results in a constitutively active JAK2, rendering cells capable of growing in a cytokine-independent manner.
TYK2 deficiency only affects Type I and Type II Interferons’ (IFNs) activity, as TYK2−/− mice showed increased susceptibility to viral and intracellular infections (14). TYK2 deficiency has also been reported in humans, albeit only two individuals have been identified so far. Interestingly, whereas one patient showed susceptibility not only to viruses and mycobacteria as expected from the mouse model, but also to fungi, and also suffered from atopic dermatitis with elevated serum IgE (15), the second patient did not have high levels of IgE, but instead suffered from disseminated Bacillus Calmette-Guerin (BCG) infection, neurobrucellosis, and cutaneous herpes zoster infection, suggesting that defective IL-12 signaling, eventually leading to impaired IFN- γ production, susceptibility to BCG and Brucella (16)
As mentioned above, among the JAKs, JAK3 is predominantly expressed in hematopoietic cells. More importantly, JAK3 only associates with the IL-2 common γ-chain receptor. This is a receptor for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. The absence of a common γ-chain results in X-linked severe combined immunodeficiency (X-SCID) (4). Boys affected with this syndrome have no T or natural killer (NK) cells and have B cells with impaired functions. Similarly, mutations in JAK3 cause autosomal recessive SCID in both boys and girls (17). The lack of T and NK cells is due to inhibition of IL-7 and IL-15 signaling. Interestingly, some JAK3–deficient patients have poorly functioning T cells and may develop autoimmune pathologies (18, 19).
The specificity of JAK3 expression within the immune compartment as well the effects of its deficiency, limited to the adaptive immune response, attracted immediate attention by the pharmaceutical industry. Similarly, as JAK2 mutations have been recognized to cause several hematologic malignancies, JAKs have become excellent targets for the development of drugs which could modulate cytokines’ effects. Twenty years after the elucidation of the pathway in which JAKs are implicated we have now reach the goal of specifically targeting this class of kinases.
JAK inhibitors
Thanks to the remarkable success of specific kinase inhibitors, inhibition of JAK signaling became a reasonable goal for the treatment of autoimmune diseases and malignancies. For example, Imatinib revolutionized the treatment of hematologic cancers and proved that targeting kinase was indeed feasible. Anti-cytokine biologics such as anti-tumor necrosis factor (TNF)α and anti-IL-6 receptor antibodies are widely used for treatment of autoimmune diseases. However, biologics have to be administered parenterally, and despite progress which now allows for subcutaneous self-administration, their route of administration is still less than ideal. Furthermore, these drugs are expensive and unaffordable for a large number of patients. Finally, in some cases (mainly rheumatoid arthritis (RA) patients), these drugs are not effective. Therefore, development of small molecules that could be administered orally and used in patients failing treatment with biologics and other disease modifying antirheumatic drugs (DMARDs) had long been desired.
After years of basic, preclinical, and clinical research, two JAK inhibitors are now Food and Drug Administration (FDA)-approved and several other drugs are in various stages of development for the treatment of rheumatoid arthritis, psoriasis, inflammatory bowel disease (IBD), and rejection of renal transplantation, as well as MPD and possibly other malignancies (Table 1).
Table 1.
JAK inhibitors in clinical development
| Target of the inhibitor | Disease in clinical trial | Completed clinical trial in US (unless otherwise specified) | ClinicalTrial.gov (USA) number | Company name | |
|---|---|---|---|---|---|
| Tofacitinib (CP690,550) | JAK1, JAK2, JAK3, | RA (appoved in US and Japan) | III | NCT00853385, NCT00814307 | Pfizer |
| Psoriasis | III | NCT01186744, NCT01241591, NCT01276639 | |||
| IBD | II | NCT00615199, NCT00787202 | |||
| Kidney Transplant Patients | II | NCT00106639, NCT00483756 | |||
| Dry Eye | II | NCT00784719, NCT01135511 | |||
| CYT387 | JAK1, JAK2 | MF | II | NCT00935987 | Cytopia |
| Baricitinib(INCB028050) | JAK1, JAK2 | RA | II | NCT00902486 | Eli Lilly (Incyte) |
| Psoriasis | II (ongoing) | NCT01490632 | |||
| Ruxolitinib(INCB018424) | JAK1, JAK2 | MF (approved in US and EU) | III (ongoing) | NCT00952289, NCT00934544 | Incyte |
| PV, ET | II/III (ongoing) | NCT01632904, NCT01243944, NCT00726232 | |||
| Psoriasis | II | NCT00820950, NCT00778700, NCT00617994 | |||
| Multiple myeloma | II | NCT00639002 | |||
| RA | II | NCT00550043 | |||
| CML, ALL, MS, AML | II (ongoing) | NCT00674479, NCT01251965 | |||
| TG101348 (SAR302503) | JAK2, FLT3 | MF | III (ongoing) | NCT01437787 | TargeGen (Sanofi-Aventis) |
| Lestaurtinib (CEP-701) | JAK2, FLT3, TrkA | MF | II | NCT00494585 | Cephalon |
| PV, ET | II | NCT00586651 | |||
| Prostate Cancer | II | NCT00081601 | |||
| AML | II | NCT00079482 | |||
| Psoriasis | II | NCT00236119, NCT00617994 | |||
| AZD1480 | JAK1, JAK2 | Solid tumor | I (development discontinued) | N/A | Astra Zeneca |
| MF | II (ongoing) | NCT00910728 | |||
| R348 | JAK3, Syk | Dry eye disease | I | NCT01733992 | Rigel |
| VX-509 | JAK3 | RA | II | NCT01052194 | Vertex Pharmaceuticals Incorporated |
| GLPG0634 | JAK1, JAK2 | RA | II | NCT01668641, NCT01384422 | Galapagos NV |
| GSK2586184 | JAK1 | SLE | I | NCT01687309 | GlaxoSmithKline (Galapagos NV) |
| AC-430 | JAK2 | RA | I | NCT01287858 | Ambit Biosciences Corporation |
| Pacritinib (SB1518) | JAK2 | MF | III (ongoing) | NCT01773187 | Wyeth |
| BMS-911543 | JAK2 | MF | II | NCT01236352 | Bristol-Myers Squibb |
| Rheumatoid arthritis | RA |
| Inflammatory bowel disease | IBD |
| Myelifibrosis | MF |
| Polycythemia vera | PV |
| Essential thrombocythemia | ET |
| Primary myelifibrosis | PMF |
| Chronic myeloid leukemia | CML |
| Acute Lymphocytic Leukemia | ALL |
| Acute Myeloid Leukemia | AML |
| Myelodysplastic Syndrome | MS |
| Chronic Myelogenous Leukemia | CML |
| Systemic Lupus Erythematosus | SLE |
Ruxolitinib (also known as INCB018424) was the first FDA-approved JAK inhibitor. Because ruxolitinib showed selectivity towards JAK1 and JAK2 (IC50 3.3nM and 2.8nM for JAK1 and JAK2 respectively, versus 19nM for Tyk2 and 323nM for JAK3), it was evaluated for its therapeutic activity in patients with MPD including primary myelofibrosis (MF), post-essential thrombocythemia MF, post-polycythemia vera MF. In various phase I, II, and III clinical trials (2, 20, 21). Ruxolutinib was shown to be efficacious, reducing spleen size in 44% of the patients receiving 15 or 25 mg of ruxolitinib twice daily, ameliorating debilitating myelofibrosis-related symptoms, and allowing overall clinical improvement in over 70% of the patients for over 12 months. Pro-inflammatory cytokines and other biomarkers were significantly decreased. The most common adverse events observed were anemia and thrombocytopenia, which mainly occurred during first 2 to 3 months of treatment. Dose reduction, dose interruption, or in case of anemia, transfusions, improved these dose-related, hematologic adverse events. Neutropenia, although less common than anemia, and thrombocytopenia has been observed and also appears to be dose-related. Interestingly, the beneficial effects of ruxolitinib seem to be independent of the JAK2 mutational status (22). Phase III trials have confirmed the positive results obtained before with improvement in splenomegaly and overall survival. However, complete molecular remission of the disease was not achieved and patients still had circulating neoplastic cells. Nonetheless, the results were such that ruxolitinib has now become the drug of choice and, it is current clinically approved for the treatment of MPD.
Ruxolitinib is also being used for other myeloproliferative malignancies such as multiple myeloma (ClinicalTrials.gov number, NCT00639002, completed phase II) and leukemia (NCT00674479 on going phase II study). Moreover, phase II studies in non-malignant conditions such as RA and psoriasis have also shown promising results (23) (NCT00550043 for RA, and NCT00820950 for psoriasis).
Importantly though, resistance to ruxolitinib and other JAK2 inhibitors has been documented (22, 24), but the mechanism of this resistance is still not completely understood. Some have reported the appearance of new mutations in the JAK2 protein similarly to what observed for resistance to other kinases inhibitors like imatinib (25). Others suggested that resistance is due to heterodimerization between JAK2 and JAK1 or TYK2 (26).
Tofacitinib (previously known as CP-690,550) was the second JAK inhibitor approved by the FDA for use in a clinical setting. Tofacitinib inhibits JAK family members with a high degree of kinome selectivity (27, 28), and was developed by Pfizer as a JAK3 inhibitor to be used as immunosuppressant in organ transplantation and possibly for the treatment of autoimmune diseases. It was soon clear that Tofacitinib inhibits not only JAK3 (IC50, 1nM) but can also inhibit JAK1 (IC50, 112nM) as well as JAK2 (IC50, 20nM) enzymatic activity (29).
Tofacitinib efficiently blocks common γ-chain cytokines including IL-2, IL-4, IL-15, and IL-21. Since both JAK1 and JAK2 are inhibited, tofacitinib also constrains signaling by IFN-γ, IL-6, and to a lesser extent IL-12, and IL-23. As a result of this rather broader activity, tofacitinib impairs differentiation of CD4+ T helper cells (Th1 and Th2), and limits the generation of pathogenic Th17 cells (30). Conversely, tofacitinib may enhance the generation of conventional Th17 cells, and it has been shown that a low dose accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation (31), although these preclinical results have not yet been confirmed in humans. As expected from the results in animals lacking JAK3 or the common γ-chain, tofacitinib also blocks NK cell differentiation. In cynomolgus monkeys, oral administration of tofacitinib resulted in reduced numbers of circulating NK cells as well as effector memory CD8+ T cells in a dose dependent manner, but preserved CD4+ T cells (32). Notably, no changes of the major CD4+ or CD8+ T cell subsets have so far been observed in clinical trials, although decreased NK cell numbers have been reported (33). Furthermore, in a mouse Th2-dependent asthma model, tofacitinib reduces pulmonary eosinophilia (34), suggesting a possible use of this drug for the treatment of allergic asthma. Tofacitinib also inhibits osteoclast-mediated bone resorption in a rat adjuvant-induced arthritis model, as well as human T lymphocyte RANKL production and human osteoclast differentiation and function, thereby inhibiting osteoclast-mediated bone resorption (35).
Remarkably, tofacitinib does not only influence the adaptive immune response, it also abrogates innate response by limiting the production of TNF and other proinflammatory cytokines in a lipopolysaccharide-induced sepsis mouse model. These results could be explained by the inhibition of IFN signaling as this particular animal model has been shown to be IFN-dependent (30). Importantly, tofacitinib inhibits activation of mouse and human macrophages and is efficacious in K/BxN serum-transfer arthritis, a model that is dependent on macrophages, but not on lymphocytes (36). These result show that tofacitinib blocks multiple steps of RA by inhibiting both innate and adaptive immunity. Moreover, in a recent study, tofacitinib appeared to be efficacious in a mouse model of celiac disease (37). Notably, the mechanism of action of tofacitinib in B cells as well as many other immune cells has not been studied yet.
The efficacy and safety of tofacitinib has been studied in multiple Phase II and Phase III clinical trials, not only for the treatment of RA but also for inflammatory bowel disease (NCT00615199) and psoriasis (NCT01186744, NCT01241591, NCT01276639), and as a potential immunosuppressant to prevent transplant rejection. The data in support of its FDA approval have recently been reported. Two Phase III trials were completed assessing the efficacy of tofacitinib in patients with RA who had failed treatment with other DMARDs. Fleischmann and colleagues enrolled patients for who failed treatment with other DMARDs (38), (NCT00814307). At month 3, ACR20 responses in the 5mg and 10mg tofacitinib-treated groups were better than those observed in the placebo group (59.8% in the 5 mg tofacitinib treated group and 65.7% in the 10 mg treated group versus 26.7% in the placebo group).
In another trial, Van Vollenhoven and his colleagues compared tofacitinib, adalimumab (an anti-TNF antibody), or placebo in patients with active disease receiving weekly methotrexate (MTX) therapy (39) (NCT00853385). At 6 months, using the American College of Rheumatology 20% improvement criteria (ACR 20), responses in patients who received 5mg and 10mg of tofacitinib were superior to those observed in the adalimumab treated group or in the placebo treated group, (51.5% in the 5mg of tofacitinib group, 52.6% in the 10mg of tofacitinib group, 47.2% in the adalimumab group, 28.3% in the placebo group, respectively).
Interestingly, the side effects of tofacitinib were similar to what had been observed in patients treated with anti-TNF antibodies such as adalimumab. Overall, the most common adverse events observed include headaches, upper respiratory infections, diarrhea, nasopharyngeal inflammation, elevation in low-density lipoprotein and cholesterol levels, and reduction of neutrophil numbers. In particular, it was pointed out that patients should be tested for latent tuberculosis before starting and during tofacitinib therapy. Other serious infections that have been reported during tofacitinib treatment include pneumonia, cellulitis, and urinary tract infections, as well as opportunistic infections such as esophageal candidiasis, pneumocystis, cytomegalovirus. The risk of incidence of herpes zoster was increased in tofacitinib-treated patients compared to the placebo treated patients and other DMARDs treated patients. Increasing the risk of malignancies and lymphomas is a common concern for RA patients with TNF inhibitors and other DMARDs. In a long-term trial, the rate of lymphomas and other lymphoproliferative disorders in tofacitinib-treated patients was 0.07 per 100 patient/years. This rate is consistent with the rate reported for RA patients treated with other DMARDs.
As mentioned above, tofacitinib is being considered as a treatment option for other autoimmune diseases. Recently, it was reported that patients with moderate to severe active ulcerative colitis treated with tofacitinib were more likely to have improved clinical response than those receiving placebo (40) (NCT00787202). Several phase III trials are also ongoing for the treatment of psoriasis patients (see Table-1). Notably, another JAK1 and JAK2 inhibitor related to ruxolitinib, baricitinib has also been found to be efficacious in patients with active RA, refractory to other DMARDs and biologics (NCT00902486).
All the inhibitors mentioned so far clearly are capable of blocking more than one JAK, albeit at different concentrations. Specific inhibitors are also being developed and appear to be quite effective. A selective JAK1 inhibitor, GLPG0634, showed effectiveness in a Phase II trial of RA patients (NCT01668641, NCT01384422). CEP-33779 is a selective JAK2 inhibitor, which has demonstrated efficacy in two preclinical models of RA. It also improved nephritis in a mouse lupus model by depleting auto-reactive plasma cells (41). Furthermore, VX-509, a JAK3 specific inhibitor with 100-fold selectivity towards JAK3 over other JAK family members, has been shown to be efficacious in a Phase II study in RA patients (NCT01052194). The efficacy, shown so far by these second-generation more specific inhibitors may indicate that the evolution of this class of drugs is still far from complete. The challenges in the future will include carefully assessing which disease will benefit more from specific JAK inhibition compared to a more broadly active drug, as well as investigating in greater detail their mechanism of action, as the possibility of resistance to kinases inhibitors has proved to be a concern.
4. Conclusions
After more than 20 years of active research by academic laboratories and the pharmaceutical industry, JAK inhibition has finally arrived. FDA approval of ruxolitinib for MPD and of tofacitinib for treatment of RA is just the beginning for this class of drugs. Targeting JAKs has been validated, and many other JAK inhibitors are rapidly moving ahead in clinical trials for other autoimmune diseases and hematologic malignancies. Despite this success, several questions remain unaddressed. How important is specificity? Inhibiting more than one JAK works well for RA and other autoimmune diseases in which multiple cytokines play a critical role. Would very specific inhibitors work better for diseases with a more specific underlying cause?
Is the kinase domain of the JAKs the only part of the molecule that could be targeted? Despite the lack of a crystal structure of a complete JAK, functional studies have shown that both the pseudokinase domain and the FERM domain are very important in controlling the enzymatic activity. How feasible would it be to target these domains, and would the mechanism of action of such compounds be different to that of current drugs?
Importantly, resistance to JAK2 enzymatic inhibition has been observed in patients treated with ruxolitinib. This has not been seen in tofacitinib-treated patients, but it is still too early to say that this will not happen. Should the use of tofacitinib be limited to patients who are unresponsive to biologics or other DMARDs? Would it be possible to switch to other JAK inhibitors and achieve remission as was done with imatinib-resistant malignancies? Ultimately all these considerations will need to be take into account in determining appropriate use of the available small molecules and those currently being developed.
Acknowledgments
We thank the following individuals for critical reading of the manuscript: Dr. Kiyoshi Hirahara, Dr. Giuseppe Sciumè, Ms. Kathryn Davis, and Dr. Jonathan Mallett.
Footnotes
Full acknowledgement and disclosures
NIAMS has a Collaborative Research and Development Agreement (CRADA) with Pfizer.
References
- 1.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28(4):477–87. doi: 10.1016/j.immuni.2008.03.002. Epub 2008/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xavier RJ, Rioux JD. Genome-wide association studies: a new window into immune-mediated diseases. Nature reviews Immunology. 2008;8(8):631–43. doi: 10.1038/nri2361. Epub 2008/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Shea JJ, Gadina M, Kanno Y. Cytokine signaling: birth of a pathway. J Immunol. 2011;187(11):5475–8. doi: 10.4049/jimmunol.1102913. Epub 2011/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annual review of immunology. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. Epub 1998/05/23. [DOI] [PubMed] [Google Scholar]
- 5.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks) Genome biology. 2004;5(12):253. doi: 10.1186/gb-2004-5-12-253. Epub 2004/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nature structural & molecular biology. 2011;18(9):971–6. doi: 10.1038/nsmb.2099. Epub 2011/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harry BL, Eckhardt SG, Jimeno A. JAK2 inhibition for the treatment of hematologic and solid malignancies. Expert opinion on investigational drugs. 2012;21(5):637–55. doi: 10.1517/13543784.2012.677432. Epub 2012/04/13. [DOI] [PubMed] [Google Scholar]
- 8.Zhou YJ, Chen M, Cusack NA, Kimmel LH, Magnuson KS, Boyd JG, et al. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Molecular cell. 2001;8(5):959–69. doi: 10.1016/s1097-2765(01)00398-7. Epub 2001/12/14. [DOI] [PubMed] [Google Scholar]
- 9.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–31. doi: 10.1016/s0092-8674(02)00701-8. Epub 2002/05/02. [DOI] [PubMed] [Google Scholar]
- 10.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278(5341):1309–12. doi: 10.1126/science.278.5341.1309. Epub 1997/11/21. [DOI] [PubMed] [Google Scholar]
- 11.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93(3):373–83. doi: 10.1016/s0092-8674(00)81166-6. Epub 1998/05/20. [DOI] [PubMed] [Google Scholar]
- 12.Van Roosbroeck K, Cox L, Tousseyn T, Lahortiga I, Gielen O, Cauwelier B, et al. JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood. 2011;117(15):4056–64. doi: 10.1182/blood-2010-06-291310. Epub 2011/02/18. [DOI] [PubMed] [Google Scholar]
- 13.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nature reviews Cancer. 2007;7(9):673–83. doi: 10.1038/nrc2210. Epub 2007/08/28. [DOI] [PubMed] [Google Scholar]
- 14.Karaghiosoff M, Neubauer H, Lassnig C, Kovarik P, Schindler H, Pircher H, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13(4):549–60. doi: 10.1016/s1074-7613(00)00054-6. Epub 2000/11/09. [DOI] [PubMed] [Google Scholar]
- 15.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25(5):745–55. doi: 10.1016/j.immuni.2006.09.009. Epub 2006/11/08. [DOI] [PubMed] [Google Scholar]
- 16.Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, Kreins AY, Grant AV, Abel L, et al. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. The Journal of pediatrics. 2012;160(6):1055–7. doi: 10.1016/j.jpeds.2012.01.056. Epub 2012/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunological reviews. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. Epub 2004/11/18. [DOI] [PubMed] [Google Scholar]
- 18.Frucht DM, Gadina M, Jagadeesh GJ, Aksentijevich I, Takada K, Bleesing JJ, et al. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes and immunity. 2001;2(8):422–32. doi: 10.1038/sj.gene.6363802. Epub 2002/01/10. [DOI] [PubMed] [Google Scholar]
- 19.Brugnoni D, Notarangelo LD, Sottini A, Airo P, Pennacchio M, Mazzolari E, et al. Development of autologous, oligoclonal, poorly functioning T lymphocytes in a patient with autosomal recessive severe combined immunodeficiency caused by defects of the Jak3 tyrosine kinase. Blood. 1998;91(3):949–55. Epub 1998/02/03. [PubMed] [Google Scholar]
- 20.Verstovsek S, Kantarjian HM, Estrov Z, Cortes JE, Thomas DA, Kadia T, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120(6):1202–9. doi: 10.1182/blood-2012-02-414631. Epub 2012/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. The New England journal of medicine. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. Epub 2012/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. The New England journal of medicine. 2010;363(12):1117–27. doi: 10.1056/NEJMoa1002028. Epub 2010/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mesa RA. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs: the investigational drugs journal. 2010;13(6):394–403. Epub 2010/05/28. [PubMed] [Google Scholar]
- 24.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(7):789–96. doi: 10.1200/JCO.2010.32.8021. Epub 2011/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigert O, Lane AA, Bird L, Kopp N, Chapuy B, van Bodegom D, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. The Journal of experimental medicine. 2012;209(2):259–73. doi: 10.1084/jem.20111694. Epub 2012/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489(7414):155–9. doi: 10.1038/nature11303. Epub 2012/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontzias A, Kotlyar A, Laurence A, Changelian P, O’Shea JJ. Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease. Current opinion in pharmacology. 2012;12(4):464–70. doi: 10.1016/j.coph.2012.06.008. Epub 2012/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nature biotechnology. 2008;26(1):127–32. doi: 10.1038/nbt1358. Epub 2008/01/10. [DOI] [PubMed] [Google Scholar]
- 29.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302(5646):875–8. doi: 10.1126/science.1087061. Epub 2003/11/01. [DOI] [PubMed] [Google Scholar]
- 30.Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690, 550) J Immunol. 2011;186(7):4234–43. doi: 10.4049/jimmunol.1003668. Epub 2011/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida H, Kimura A, Fukaya T, Sekiya T, Morita R, Shichita T, et al. Low dose CP-690,550 (tofacitinib), a pan-JAK inhibitor, accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation. Biochem Biophys Res Commun. 2012;418(2):234–40. doi: 10.1016/j.bbrc.2011.12.156. Epub 2012/01/19. [DOI] [PubMed] [Google Scholar]
- 32.Conklyn M, Andresen C, Changelian P, Kudlacz E. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J Leukoc Biol. 2004;76(6):1248–55. doi: 10.1189/jlb.0504282. Epub 2004/09/17. [DOI] [PubMed] [Google Scholar]
- 33.van Gurp E, Weimar W, Gaston R, Brennan D, Mendez R, Pirsch J, et al. Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(8):1711–8. doi: 10.1111/j.1600-6143.2008.02307.x. Epub 2008/06/19. [DOI] [PubMed] [Google Scholar]
- 34.Kudlacz E, Conklyn M, Andresen C, Whitney-Pickett C, Changelian P. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. European journal of pharmacology. 2008;582(1–3):154–61. doi: 10.1016/j.ejphar.2007.12.024. Epub 2008/02/05. [DOI] [PubMed] [Google Scholar]
- 35.Onuora S. Experimental arthritis: JAK inhibition with tofacitinib curbs RANKL-induced joint damage. Nat Rev Rheumatol. 2012;8(10):564. doi: 10.1038/nrrheum.2012.147. Epub 2012/09/05. [DOI] [PubMed] [Google Scholar]
- 36.Rosengren S, Corr M, Firestein GS, Boyle DL. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis. 2012;71(3):440–7. doi: 10.1136/ard.2011.150284. Epub 2011/11/29. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama S, Perera PY, Waldmann TA, Hiroi T, Perera LP. Tofacitinib, a Janus Kinase Inhibitor Demonstrates Efficacy in an IL-15 Transgenic Mouse Model that Recapitulates Pathologic Manifestations of Celiac Disease. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9849-y. Epub 2012/12/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. The New England journal of medicine. 2012;367(6):495–507. doi: 10.1056/NEJMoa1109071. Epub 2012/08/10. [DOI] [PubMed] [Google Scholar]
- 39.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. The New England journal of medicine. 2012;367(6):508–19. doi: 10.1056/NEJMoa1112072. Epub 2012/08/10. [DOI] [PubMed] [Google Scholar]
- 40.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. The New England journal of medicine. 2012;367(7):616–24. doi: 10.1056/NEJMoa1112168. Epub 2012/08/17. [DOI] [PubMed] [Google Scholar]
- 41.Lu LD, Stump KL, Wallace NH, Dobrzanski P, Serdikoff C, Gingrich DE, et al. Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. J Immunol. 2011;187(7):3840–53. doi: 10.4049/jimmunol.1101228. Epub 2011/09/02. [DOI] [PubMed] [Google Scholar]