Abstract
As the rational application of targeted therapies in cancer supplants traditional cytotoxic chemotherapy, there is an ever-greater need for a thorough understanding of the complex machinery of the cell and an application of this knowledge to the development of novel therapeutics and combinations of agents. Here, we review the current state of knowledge of the class of targeted agents known as cyclin-dependent kinase (CDK) inhibitors, with a focus on chronic lymphocytic leukemia (CLL). Flavopiridol (alvocidib) is the best studied of the CDK inhibitors, producing a dramatic cytotoxic effect in vitro and in vivo, with the principal limiting factor of acute tumor lysis. Unfortunately, flavopiridol has a narrow therapeutic window and is relatively non-selective with several off-target (i.e. non-CDK) effects, which prompted development of the second-generation CDK inhibitor dinaciclib. Dinaciclib appears to be both more potent and selective than flavopiridol, with at least an order of magnitude greater therapeutic index, and is currently in phase III clinical trials. In additional to flavopiridol and dinaciclib, we also review the current state of other members of this class, and provide commentary as to the future direction of combination therapy including CDK inhibitors.
Keywords: Pharmacotherapeutics, Chemotherapeutic approaches, Cell cycle and apoptosis changes, Lymphoid Leukemia, Lymphoma and Hodgkin disease
Introduction
It has been recognized for more than twenty years that cyclins are important regulators of cell cycle in both normal and transformed eukaryotic cells [1,2]. In some hematologic malignancies, dysregulation of cyclin (for example, the constitutive expression of cyclin D1 under the control of the immunoglobulin heavy chain promoter in mantle cell lymphoma) is believed to be a primary oncogenic driver, while in other diseases dysfunctional cyclin may be a result of an upstream process. Cyclins in turn regulate so-called cyclin-dependent kinases, which have a variety of function within the cell including and in addition to their eponymous role in the regulation of cell cycle. Because control of cell cycle is an attractive target in anti-cancer therapy, inhibitors of cyclin-dependent kinase (CDK) have been explored as a novel therapeutic class. Although control of cell-cycle was a primary motivator for the development of CDK inhibitors, recent investigations into the mechanisms of CDK inhibitor-mediated cytotoxicity have highlighted roles of the cyclins and CDKs unrelated to cell cycle but important in the maintenance of the cancer cell.
CDK inhibitors have been studied in a variety of diseases, but their efficacy has been most appreciated in chronic lymphocytic leukemia (CLL). For this reason the rest of this review will focus primarily on the CDK inhibitors in CLL, with reference made to other leukemias and lymphomas as appropriate.
Cyclins fluctuate with cell cycle and regulate CDKs
The eukaryotic cell cycle results from a complex interplay of extrinsic circulating growth factors, neighborhood anti-growth stimuli, signaling networks, and internal positive and negative regulators of DNA duplication and cell division. Independence of growth factors and insensitivity to anti-growth signals, described by Hanahan and Weinberg as two of the hallmarks of cancer [3], imply unchecked progression through cell cycle checkpoints and consequent loss of control of cell cycle.
The discovery of cyclins, a novel class of proteins whose expression fluctuates regularly inside the cell, provided the first clues about the biochemical workings of the cell cycle [2]. In the normal, non-quiescent eukaryotic cell, the regular rise-and-fall of cyclins is integral to the orderly progression through the cell cycle. Cyclins do not act alone, however, and require the presence of a CDK to do their regulatory work. Typically, each cyclin has one or two CDKs with which it associates, and each CDK has one or two cyclins with which it associates. Cyclins (the regulatory unit) in combination with their partner CDK (the catalytic effector unit) are responsible for phosphorylating other cell cycle regulatory proteins [4]. One of the best-characterized interactions is that of CDK4/Cyclin D with members of the retinoblastoma (Rb) family: Rb proteins sequester E2F family transcription factors, but phosphorylated-Rb dissociates from E2F family transcription factors, thereby permitting transcription [1,5,6]. In this way, CDK4/Cyclin D leads to transcription of Cyclin E, which further represses Rb and advances the cell through mitosis [2]. A partial list of CDKs and their associated cyclins can be found in table 1.
Table 1.
Selected CDK/cyclin targets.
| CDK | Cyclin Partner(s) | Function |
|---|---|---|
| CDK1 | Cyclin A, B | G2 phase; M phase |
| CDK2 | Cyclin A, E | S phase |
| CDK4 | Cyclin D* | G1 phase |
| CDK5 | Cyclin D*, E, G | Senescence |
| CDK6 | Cyclin D* | G1 phase |
| CDK7 | Cyclin H | Transcription (RNAP II) initiation |
| CDK8 | Cyclin C | Mediator complex; Beta-catenin modulation |
| CDK9 | Cyclin T**, K | Transcription (RNAP II) elongation |
human cyclin D takes three isoforms: D1, D2, D3
human cyclin T isoforms T1, T2a, and T2b
CDKs have a variety of functions
The CDKs are a family of serine/threonine protein kinases that were first discovered to play a key part in the regulation of procession through the cell cycle via phosphorylation and inactivation of Rb, the retinoblastoma tumor suppressor gene product [2,6,7]. It was subsequently discovered that a large variety of cyclins in complex with CDKs were pivotal in all transition points in the cell cycle, including the G2/M checkpoint. For cancers whose expansion is driven by rapid, out of control proliferation, inhibition of those CDKs that permit progression through the cell cycle is a natural and attractive target, bolstered by in vitro evidence that loss of CDK1 and CDK2 activity demonstrably arrests the cell cycle and induces apoptosis in normal and malignant human cells [8–10].
However, not all CDKs control progression throughout the cell cycle: other members of the CDK family take on other roles, and the title cyclin dependent kinase then alludes to the cell cycle somewhat misleadingly in that these proteins perform in roles seemingly unrelated to cell cycle. For example, CDK5 has a role in neuronal function and tau phosphorylation [11], while CDK7, CDK8, and CDK9 function in regulation of transcription [12,13]. The highly conserved CDKs 7, 8, and 9 play key roles in RNA transcript production through initiation and sustenance of RNA polymerase II (RNAP II) mediated transcription via phosphorylation in the carboxy-terminal domain of RNAP II. While CDK7-effected phosphorylation of RNAP II permits initiation of transcription, CDK9 and its cyclin partners (T1, T2a, T2b and K) act together to positively promote elongation of existing transcripts [14]. Thus, one strategy in indolent cancers (wherein tumor mass is a result of enhanced survival more so than unchecked proliferation) could be to shut off messenger RNA transcription by inhibition of CDK7 and CDK9. So long as transcription could be repressed, the level of short-lived proteins should then decline. Indeed, anti-apoptotic proteins of the Bcl2 family (Bcl2, Bax, Mcl-1, Bcl-XL, XIAP, and others) are overexpressed in CLL cells, are a putative cause of these cells’ extended survival [15,16], and therefore represent a potential target [17–20].
Specific agents
Although a number of CDK inhibitors have entered into clinical trials, by far the most thoroughly studied is the synthetic flavone flavopiridol; consequently the majority of studies reviewed here involve flavopiridol. More recently, the newer CDK inhibitor dinaciclib appears promising and has entered phase II and III clinical trials. Other compounds are in clinical development, and some of these are surveyed briefly. A partial listing of CDK inhibitors under investigation is included in table 2.
Table 2.
Cyclin dependent kinase inhibitors in clinical development.
| Agent (alternative names) | Targets | Status* |
|---|---|---|
| Alvocidib (flavopiridol, HMR-1275, L86-8275, NSC-649890) |
CDKs 1,2,4,6,7,9 | Phase II |
| BAY-1000394 | CDKs 1𠄴,7,9 | Phase I |
| Dinaciclib (MK-7965, SCH-727965) | CDKs 1,2,5,9 | Phase III |
| P276-00 | CDKs 1,4,9 | Phase II |
| R547 | CDKs 1,2,4 | Phase I completed |
| Seliciclib (R-roscovitine, CYC-202) | CDKs 1,2,5,7 | Phase II terminated |
| SNS-032 (formerly BMS-387032) | CDKs 2,7,9 | Phase I completed |
| TG02 | CDKs 1,2,7,9 FLT3, JAK2 | Phase I |
based on trials registered at http://clinicaltrials.gov and https://www.clinicaltrialsregister.eu/
Flavopiridol
Flavopiridol is a semi-synthetic flavone derived from rohitukine alkaloid extracts of Dysoxylum binectariferum, a tree native to India and closely related to the Ayurvedic plant D. malabaricum, used for the treatment of rheumatoid arthritis [21]. First described as a potential anti-neoplastic agent in 1992, it was initially thought to be cytostatic only, inducing cell-cycle arrest in dividing cells through competitive inhibition of CDK2, but later experiments confirmed the ability to induce apoptosis in quiescent cells [5,22–24]. Subsequent investigations expanded the known spectrum of flavopiridol’s activity: CDK1, CDK2, and CDK4 were all confirmed to be inhibited by competitive binding at the ATP-binding pocket [5,22,25]. Flavopiridol also appears to inhibit CDK9, and consequently transcription, through similar ATP-competitive inhibition [26–28].
In preclinical studies, it was quickly recognized that flavopiridol had activity in human tumor cell-lines, and B-cells in particular [29–32]; early phase clinical trials in humans soon followed. Among the CDK inhibitors, flavopiridol is heretofore the most comprehensively studied, and of the diseases in which it has been fielded the most promising work has been in the area of CLL [33], likely due to a few particular biologic effects of CDK inhibition which are relevant to the oncogenic underpinnings of the CLL cell.
Early work with flavopiridol in human tumor cell lines demonstrated drug-mediated cell-cycle arrest and apoptosis [34], but even more remarkably flavopiridol also seemed to induce apoptosis in non-cycling (i.e. G0–1 phase) cancer cells [23,24]. In distinction to other leukemias in which the primary problem is rapid, out-of-control proliferation, in CLL a large fraction of the numerous lymphocytes are not actively cycling; the principal driver of the increase in tumor mass is instead believed to be cells’ evasion of apoptosis. Flavopiridol, demonstrably capable of inducing apoptosis in non-cycling tumor cells thus seemed an ideal agent to try in CLL. Early studies by several groups demonstrated that despite the non-proliferating nature of CLL cells, flavopiridol mediated robust early apoptosis in vitro whereas the effect of this agent against normal T-cells was minimal [29–32,35,36]. Additionally, flavopiridol favorably down-modulated several anti-apoptotic Bcl-2 family proteins and promoted cell death independent of p53. The induction of p53-independent apoptosis strengthened flavopiridol’s importance in CLL, a disease in which the complete lack of p53 (the del(17p) cytogenetic abnormality) portends particularly poorly [37,38]. Subsequent studies by Chen and Plunkett [39] demonstrated that flavopiridol also inhibits CDK9 in CLL cells, in turn decreasing phosphorylation of RNA polymerase II, ultimately depleting several short half-life proteins such as Mcl-1 that protect CLL cells from apoptosis (figure 2). Additionally, recent studies with flavopiridol by our group have demonstrated this agent promotes reduction of mitochondrial oxygen consumption and autophagy [40]. Detailed interrogation of autophagy in a subsequent study demonstrated this process to actually be a protective mechanism against flavopiridol-mediated death [41]. Notably in this study we were able to demonstrate that flavopiridol also promoted robust endoplasmic reticulum (ER) stress with downstream activation of ASK1 and caspase 4; inhibition of caspase 4 as well as siRNA knock down of ASK1 prevented apoptosis mediated by flavopiridol. Documentation of activation of ER stress in vivo with flavopiridol in this paper further supported this as a very relevant new mechanism of action of flavopiridol mediated cell death in CLL. Further study of this novel mechanism of action is underway at this time with both flavopiridol and other second-generation inhibitors.
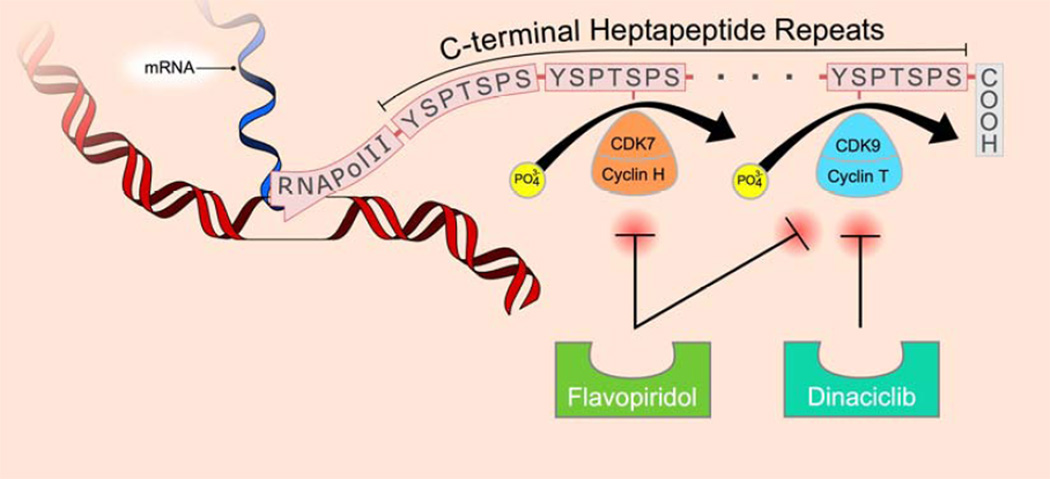
Figure 2.
Pro-survival (anti-apoptotic) mediators such as Mcl-1 have a short half-life and are actively transcribed in some cancer cells by RNA polymerase II, which requires phosphorylation at serine 2 and serine 5 within the heptapeptide repeats that comprise its C-terminal domain (CTD). Cyclin-dependent kinases 7 and 9 phosphorylate these serines, but can be inhibited by cyclin-dependent kinase inhibitors, ultimately leading to decreased levels of anti-apoptotic proteins and therefore increased cell death.
Flavopiridol clinical trials
In the first phase I clinical trial to be published, Senderowicz and colleagues reported on flavopiridol given as a continuous intravenous infusion (CIVI) for 72 hours every 2 weeks in seventy-six patients with advanced refractory neoplasms [42]. The initial dose-limiting toxicity was diarrhea, and the maximum tolerated dose (MTD) without antidiarrheal prophylaxis was 50 mg/m2/d × 3 days. However, when cholestyramine and loperamide were used as antidiarrheal prophylaxis an MTD of 78 mg/m2/d × 3 days was attainable. Because of the relative frequency of diarrhea in early studies, and because a mean flavopiridol concentration 271 nmol/L (within the range of efficacy in earlier in vitro studies [29]) was achieved in the 50 mg/m2 group, this was put forward as the recommended phase 2 dose. Unfortunately, several subsequent phase II studies using this dose and schedule failed to demonstrate clinical benefit in patients with a variety of relapsed or refractory cancers, including mantle cell lymphoma [43–47]. Similarly, in CLL, no clinical efficacy was found in phase II clinical trials of either a 24-hour (80 mg/m2) or 72-hour CIVI of flavopiridol [48,49]. However, a second arm in the CALGB 19805 study used a 1-hour bolus of 50 mg/m2 daily for 3 days based upon leukemia xenograft studies showing benefit to this new schedule [31], and was able to show modest benefit: 11% partial response and 53% stable disease, compared to zero and 27%, respectively, in the 72-hour CIVI arm of the same trial [49].
In light of these disappointments, and in the context of earlier experiments showing markedly differential binding of free drug to human plasma proteins compared to the fetal calf serum typically used in in vitro studies [50], estimates of the 1-hour and 24-hour LC50 for flavopiridol were revised markedly higher: 3510 nmol/L and 470 nmol/L, respectively–levels that were definitely not achieved with current dosing strategies. The earlier failures were thus attributed to failure to achieve a therapeutic drug concentration with the 72-hour CIVI strategy.
With the foregoing in mind and with a goal to achieve biologically relevant drug concentration in vivo, our group used pharmacokinetic (PK) modeling to design a dosing schedule wherein a 30-minute loading dose was followed by a 4 hour infusion of flavopiridol every week for 4 of 6 weeks, with a target plasma concentration of > 1.5 µmol/L [36]. Fifty-two patients (43 with relapsed CLL and 9 with relapsed small lymphocytic lymphoma, SLL) in four cohorts were enrolled on a phase I study using this strategy with tolerability and PK data as endpoints [36,51]. In cohorts three and four, intrapatient dose escalation was permitted on cycle 2 day 1 and cycle 1 day 8, respectively. Retreatment of previous responders was permitted; six with a response to initial study therapy were retreated after progression of disease. Overall, the pretreatment characteristics portended poorly: at enrollment, forty-two (81%) of patients were Rai stage III or IV, the median number of prior therapies was 4, fifty-one (98%) had been exposed to fludarabine with forty-three (83%) of them being refractory to their latest fludarabine, thirty-eight (73%) had bulky lymphadenopathy, and forty patients (77%) had high risk cytogenetic features.
Eclipsing the previously described diarrhea as the most common grade 3 toxicity was acute tumor lysis syndrome (TLS), which was a dose limiting toxicity in the second dose level resulting in death of a patient due to hyperkalemia prior to the time dialysis could be initiated. This led to temporary study suspension and subsequent introduction of aggressive early monitoring as well as assurance of ability to initiate dialysis within 60 minutes of appreciation of hyper-acute tumor lysis. Through the remaining portion of the study, TLS was observed to some degree in 30 patients (57%). With application of stepped up dosing, aggressive prophylaxis, monitoring, and exclusion of patients with white blood cell count (WBC) greater than 200×103/µL, the drug could then safely be administered with close monitoring. Other common toxicities included diarrhea, transient transaminitis, and an interesting constellation of toxicities including fatigue/malaise, fever, and local tumor pain that was subsequently characterized as a cytokine release syndrome (CRS) [52].
PK analysis of 51 patients was performed in this study with a primary endpoint to reach and sustain a plasma concentration of greater than or equal to 1.5 µmol/L for the duration of the 4-hour infusion. Forty-five patients achieved or exceeded the target peak concentration, but only 20 maintained this concentration for ≥ 4 hours. Across response groups (PR vs. SD vs. PD), only area under the curve (AUC) was statistically significant at the p ≤ 0.05 level, although other PK parameters (Cmax, CL) showed a trend toward significance.
Impressively, of 52 patients receiving therapy, 21 (40%) achieved a PR with an overall median progression free survival (PFS) of 12 months. Two additional patients with WBC > 200×103/µL had 89 and 98% drops in white count, but were excluded from the responders due to having only received one cycle of therapy. Although all 21 patients eventually relapsed, 6 were re-treated, with an encouraging 5/6 achieving another PR which was itself relatively durable: the median PFS after re-treatment was another 10 months (range 6.5–19.1).
Encouraged by these findings, our group conducted a phase II trial of flavopiridol in patients with relapsed or refractory CLL [53]. The previously successful bolus plus infusion strategy was used at an initial dose of 30 mg/m2 IV bolus + 30 mg/m2 CIVI × 4 hours for the first dose, with escalation of the infusional dose to 50 mg/m2 CIVI × 4 hours for the second and subsequent doses if the patient did not experience severe TLS on cycle 1 day 1. Sixty-four patients enrolled and the overall patient characteristics were similar to the phase I trial with respect to number of prior therapies, exposure to purine nucleoside analogues, and bulky lymphadenopathy. With vigilance for TLS and the enrollment criterion that WBC be fewer than 200×103/µL, only 3 patients needed hemodialysis. As with prior studies, cytokine release syndrome was a common feature until 20 mg of IV dexamethasone prior to flavopiridol was instituted as a preventative measure. With this intervention, the rate of CRS decreased from 56 to 22%. In addition to dexamethasone prophylaxis, the study was amended to increase tolerability by shortening the cycle length from six weeks to four, and decreasing the number of weekly treatments from four to three. Pegfilgrastim was added in the third week. Overall response rate in this study compared favorably with the previous phase I trial: 34 patients (53%), with 30 (47%) PRs, 3 (5%) nodular PRs, and 1 CR. Seven of the 34 responders (21%) were able to proceed to allogeneic stem cell transplant due to a reduction of bulky lymphadenopathy. Median PFS for all patients was 8.6 months, for responders 12 months, and 10 months for those responders who did not go on to receive transplant. Historically poor-risk predictors, including del(17p13.1), del(11q22.3), complex karyotype, elevated β2-microglobulin, and bulky lymphadenopathy were not predictive in this study: responses (the primary endpoint) as well as the secondary endpoint PFS were similar irrespective of high-risk features. Indeed, a majority of the high-risk patients experienced a response. This flavopiridol trial demonstrated not only efficacy, but also that with appropriate monitoring and prophylaxis, TLS can be prevented or managed, and that with dexamethasone premedication, the CRS symptoms can be ameliorated.
Because CLL is a disease of older adults (with a median age of 72 at diagnosis), any new therapy would ideally be well tolerated in the elderly, yet these patients remain poorly represented in cancer clinical trials. We sought to know how older patients with CLL fared in the previous two trials [54]. Retrospective review of data from these two trials demonstrated that among patients seventy and older (21% of a total of 116 patients) there was no difference in response rate or PFS when compared to patients under seventy years. Overall survival was slightly worse at 2.1 years versus 2.4 years, but this difference disappeared when adjusted for other factors (for example, older patients were more likely to have a complex karyotype). Unlike fludarabine [55], flavopiridol appears safe and effective in older adults.
On the basis of this positive single-center experience, a large international multi-center study of flavopiridol in relapsed and refractory CLL was initiated and accrued 165 patients. A preplanned interim analysis of the data was presented at the American Society of Hematology (ASH) 2010 annual meeting [56] with 68 patients (41% of planned accruals) having completed at least two cycles. Using the rationally designed “bolus plus infusion” strategy given weekly for four weeks followed by a two week break, investigators were able to show as much as 31% objective response rate, though all responses were PR. Only nine percent of patients progressed. Among the responders, the responses were again durable: median PFS was about one year (12.2 months). Sixteen and twenty evaluable patients had del(17p13.1) and del(11q22.3), respectively, and objective responses were as high as 25% and 30% in these two groups. Patients with bulky lymphadenopathy (about two thirds of evaluable patients) also benefitted, with 32–39% (depending on criteria) of patients responding. Toxicities were similar to previous trials, and CRS and TLS were effectively ameliorated with corticosteroids and prompt hemodialysis. The response noted in this study matches that observed by OSU investigators using this non-optimized schedule of administration (i.e., four weekly doses in a six week cycle). Further efforts to develop flavopiridol as a monotherapy in CLL should utilize the three-week schedule with steroid prophylaxis identified in our phase II study.
Flavopiridol in combination
Based upon the observation that flavopiridol downregulates Mcl-1 [35], an antiapoptotic protein responsible in part for fludarabine and rituximab resistance, Lin et al. in a phase I trial combined flavopiridol with fludarabine and rituximab for patients with mantle cell lymphoma (MCL) and indolent B-cell malignancies [57]. Fludarabine and Rituximab were given in the standard fashion (fludarabine 25 mg/m2 daily for five days and rituximab 375 mg/m2 on day 1, on a 28-day cycle) with the addition of flavopiridol in one of four schedules, two of which were of the bolus plus CIVI type as described above. Of a total of 38 patients (10 with MCL, 9 with follicular lymphoma, 4 with marginal zone lymphoma, 1 with lymphoplasmacytic lymphoma, 3 with SLL, and 11 with CLL), 31 (82%) had a response, while 21 (55%) had a complete response; responses were independent of prior treatment. Median progression free survival was relatively durable at 25.6 months.
A shown in preclinical studies, flavopiridol exerts its lymphocyte killing effect in a manner independent of p53 status. Because alterations in TP53 play an important role in the outcomes of B-cell leukemias and lymphomas [37,58], the combination of flavopiridol with other agents that act independently of p53 has theoretical therapeutic potential. The immunomodulator lenalidomide, though incompletely understood, may enhance T-cell mediated killing of malignant B lymphocytes and has shown promise in heavily pretreated CLL patients with adverse prognostic features [59]. To test its synergy with flavopiridol, we conducted a phase I study of flavopiridol and lenalidomide in patients with relapsed CLL [59]. Weekly flavopiridol bolus plus infusion was combined with escalating doses of daily lenalidomide each for three weeks with a two-week break (cycle length 35 days). Data were last reported at the 2011 ASH annual meeting. At that time, 23 patients had completed one or more cycles of therapy and were evaluable. A PR was observed in 13 patients (57%), among them 7 with del(17p13.1) and 6 with del(11q22.3), 9 with complex cytogenetics, 5 who were fludarabine-refractory, and 6 with bulky lymphadenopathy. Six patients were able to proceed to transplant, and four of them remain in remission. Median PFS was 7 months (with a range of up to 24 months) and median OS was 23 months. Toxicities were manageable, with only two patients requiring hemodialysis and there was minimal tumor flare with steroid prophylaxis. Flavopiridol and lenalidomide appear to be a safe and effective combination that should be explored in larger studies.
Bortezomib, a proteasome inhibitor, is another novel therapy that has shown activity in a variety of hematologic cancers, presumably through inhibition of over-expressed and constitutively activated cell cycle regulators and transcription factors, including cyclins, Bcl-2 family proteins, and NF-κB. The combination of bortezomib with CDK inhibitor has been studied and has shown in vitro activity in both lymphoid and myeloid cell lines with (among other effects) diminished expression of Bcl-2 family proteins and decreased Akt and STAT family activity [60,61]. Early results in humans have been equally as promising: results of a phase I trial of bortezomib in combination with flavopiridol in patients with Non-Hodgkin Lymphoma (NHL; 9 patients) and plasma cell dyscrasias (7 patients) were notable for a number of complete responses (2/16; 12%) as well as PR (5/16, 31%), all at the MTD [62]. As this was a phase I dose-finding study, future study is warranted.
Flavopiridol pharmacokinetics
Average peak serum concentration of 271 nmol/L was achieved in early studies of a 50 mg/m2 given as a 72-hour continuous infusion. Although this was above the predicted LC50 for flavopiridol, lack of clinical response prompted further investigation which revealed that differential drug binding to fetal calf serum versus human serum meant that actual LC50 for flavopiridol was much higher. With a target of 1500 nmol/L (1.5 µmol/L) sustained for four hours, a bolus plus infusion strategy was designed. With this new strategy, serum drug concentrations easily exceeded this target (AUC range 4.51 to 31.4 µM/h), even at the lower dose levels [36].
Flavopiridol resistance
Although traditional risk factors do not predict response to flavopiridol, and although initial response is often brisk, patients do relapse. As a class of drugs, mechanisms of resistance to CDK inhibitors have so-far been poorly described, although in 2012 our group described autophagy as a novel mechanism of resistance for flavopiridol [41]. Future studies are warranted, especially in the typically very refractory population of flavopiridol patients.
Flavopiridol looking forward
Flavopiridol showed early promise as a single agent, but the paucity of complete responses prompted a search for combinations to increase its effectiveness. Combination both with traditional cytotoxic chemoimmunotherapy as well as with other novel agents has demonstrated the ability to produce CR in patients even with very advanced disease, with the caveat that there seems to be a fine line between ineffectiveness and life-threatening tumor lysis. Flavopiridol’s strengths and limitations are informing the design of future drugs and therapeutic combinations.
Dinaciclib
Studies with flavopiridol have been encouraging, but limited due in part to the narrow therapeutic window. Efforts to overcome this limitation and develop a CDK inhibitor with a broader therapeutic index have seen early success in dinaciclib (SCH-727965, later MK-7965), a compound developed using a traditional drug-screening program with permutations on a basic scaffold assessed for tumor response in an in vivo model with simultaneous calculation of the therapeutic index, taking flavopiridol as a benchmark [63]. The result of these efforts is a newer potent and selective inhibitor of CDKs with a therapeutic index in initial mouse studies more than ten times that of flavopiridol [63]. Although dinaciclib is specific for CDKs (whereas flavopiridol affects a broader range of serine/threonine and tyrosine kinases), there is considerable structural homology in the CDK family and for that reason dinaciclib, like other current-generation CDK inhibitors, exerts its effects through a specific combination (likely different from drug-to-drug) upon various CDKs; in this case, inhibition of CDKs 1, 2, 5, and 9 with IC50 of 3, 1, 1, and 4 nmol/L, respectively [64].
Dinaciclib, like flavopiridol, induces apoptosis in CLL cells irrespective of traditionally negative prognostic factors such as prior fludarabine exposure, unmutated IGHV, or del(17p13.1) status [65]. This is tremendously advantageous, as discussed above under flavopiridol. Dinaciclib-mediated cytotoxicity is not inhibited by cytokines which otherwise prevent spontaneous apoptosis in CLL cells: CD40 ligand, BAFF, TNF-alpha, and IL-4. Overcoming these survival signals seems to be through down-regulation of the respective receptors. Dinaciclib is ineffective, however, at overcoming the protective effect of direct contact of CLL cells with stromal cells. Notably, the PI3K inhibitor PIK-75 (an alpha isoform specific inhibitor) is able to abrogate the protective effect of the stromal environment in dinaciclib-treated CLL cells, suggesting a potential rational combination of targeted therapies [65].
Dosing and administration
Two alternative schedules have been described with dinaciclib. First, a 2-hour I.V. infusion on days 1, 8, and 15 of a 28-day cycle was explored in phase I studies with expansion into a phase II study of patients with indolent and large cell lymphomas [66]; the recommended phase II dose in this case was 12 mg/m2, although two patients (12.5%) required de-escalation to 10 mg/m2 because of toxicity [66]. The schedule has been used successfully in CLL patients, although in this case the maximally tolerated dose was 14 mg/m2 [67]. Second, a much larger dose (up to 50 mg/m2) given by 2-hour I.V. infusion once every 21 days has been used in acute leukemia. This 50 mg/m2 dose and 21-day schedule is based on safety and tolerability data from a trial of dinaciclib in 81 patients with advanced malignancies, including solid tumors [68]. Interestingly, this study also explored dose-limiting toxicities in extended 8-hour and 24-hour infusions, finding acceptable safety profile at 7.4 and 10.4 mg/m2 for 8- and 24-hour infusions, respectively, which may have relevance in light of data regarding transience of Mcl-1 inhibition, described below.
Clinical trials in CLL
Although dinaciclib has entered into phase II clinical trials for CLL, these data have yet to be published. An update on the phase I study of dinaciclib in patients with relapsed or refractory CLL made at the 2011 ASCO annual meeting reported on a total of 33 patients in five dose cohorts, 16 of whom were enrolled as an expansion cohort at the maximally tolerated dose of 14 mg/m2 [69]. Partial response by international working-group criteria [70] was observed in 15 of 33 patients (45%), fourteen of whom had prior fludarabine exposure. Fifteen patients had del(17p13.1), and seven (47%) achieved PR. Of all those who did respond, responses were observed at all dose levels and both early (after 2 cycles) as well as late (after 6 cycles) in treatment. When considering the sixteen patients who received the maximally tolerated dose (the recommended phase 2 dose) of 14 mg/m2, the PR rate increased to 62.5%. Among all thirty-three patients, twenty-four remained on study at the time of analysis with a follow up period ranging from 3 to 73 weeks. The median PFS was not yet reached. In addition, the majority of those with active, proliferative disease at the time they began therapy experienced stability of disease while on treatment. As expected, 25 of 27 patients with correlatives performed in this study had a sharp decline in the level of the antiapoptotic protein Mcl-1. The half-life (t1/2) of the drug was calculated as 2.1 to 3.8 hours. Toxicities echoed strongly those of flavopiridol, including diarrhea and hematologic toxicity manifested in all cell lines (in one case perhaps contributing to a DLT of bacterial pneumonia); most notably five patients experienced TLS, two of whom required dialysis (one each at the MTD of 14 mg/m2 and the maximum administered dose of 17 mg/m2). Overall, this study provided a good rationale for future clinical trials of dinaciclib in CLL, especially in patients with prior fludarabine exposure or del(17p13.1), as neither of those traditionally poor-risk factors seemed to influence response.
Clinical trials in lymphoma
A phase II clinical trial of dinaciclib in sixteen patients with indolent and large cell lymphoma utilized the 12 mg/m2 dose and days 1, 8, and 15 every 28 days schedule established in phase I trials of solid tumor patients [66]. Four patients (25%) have had measurable activity: one patient with diffuse large B-cell lymphoma experienced a partial response good enough to proceed to autologous hematopoietic cell transplant, while three patients experienced activity not meeting criteria for partial response by decrease in size of nodes. The commonest severe toxicities were hematologic.
On the basis of the foregoing positive results, dinaciclib as a single agent is now in phase III clinical trials in patients with refractory CLL and SLL and will be compared in a randomized fashion with ofatumumab. Earlier phase studies of dinaciclib in combination therapy are ongoing.
Other agents
A partial list of cyclin-dependent kinase inhibitors is given in table 2, along with their structures and current status. We will survey briefly some of these drugs, bearing in mind that at this time few agents apart from flavopiridol and dinaciclib have undergone much scrutiny in human trials. Information about the current status of clinical trials was gathered from http://clinicaltrials.gov and https://www.clinicaltrialsregister.eu/.
BAY-1000394 is an oral pan-CDK inhibitor which has shown activity against human tumor xenografts in mice, and has now entered early phase clinical trials [71].
P276-00, like flavopiridol, is a synthetic flavone which inhibits CDKs, but with less off-target inhibitor effect on non-CDK kinases, and with particular selectivity for the CDK4/Cyclin D1, CDK1/Cyclin B, and CDK9/Cyclin T1 complexes [20,72–74]. Preclinical studies of P276-00 have demonstrated decrease in phosphorylated Rb (pRb), seemingly confirming CDK4/Cyclin D1 inhibition, and these observations have formed the basis for 10 clinical trials in Cyclin D1-driven or expressing solid tumors and hematologic cancers, five of which remain active. An early report suggests safety and tolerability are good, achieving concentrations adequate for target inhibition [75].
R547 is a selective inhibitor of CDK1, CDK2, and CDK4 at physiologically relevant doses, and results from a single phase Ia clinical trial in patients with advanced cancers was reported at the 2007 American Society of Clinical Oncology (ASCO) annual meeting [76–78]. This study demonstrated drug concentrations predictive of efficacy in preclinical models, with mostly mild, non-hematologic toxicities. Enthusiasm for the compound has been limited, however, and no additional studies have been registered or reported.
Seliciclib (CYC-202, R-roscovitine) is an oral purine analogue which inhibits CDK2 strongly (mediated by ATP-competitive binding), as well as a number of other CDKs with less potency, including CDK1, CDK5, and CDK7 [79–82]. Roscovitine has shown activity in vitro against a number of human tumor cell lines with evidence of death by apoptosis [83,84]. As CLL cells are dependent upon constitutive expression of anti-apoptotic proteins, this provides an attractive target. In a preclinical study of roscovitine, Hahntow and colleagues demonstrated caspase-mediated apoptosis in 21 of 28 CLL samples [85]. As a potential explanation for this apoptosis, the anti-apoptotic proteins Mcl-1 and X-linked Inhibitor of Apoptosis (XIAP), but not Bcl-2, were down regulated. Relevant clinically, apoptosis was induced irrespective of Zap-70 status, and at concentrations that did not kill normal B-lymphocytes.
Seliciclib (the R-isomer of roscovitine) was further tested against a panel of 26 CLL samples (11 with mutations in either ATM or TP53) with similar results [86]. Within 24 hours, all 26 samples exposed to CYC-202 had experienced apoptosis, independent of p53. Down regulation of Mcl-1, but not Bcl-2 was observed in line with previous studies and probably due to the latter’s longer half-life. They further demonstrated that the decrease in the level of transcripts was due to decrease in activation of RNAP II, providing indirect evidence that seliciclib inhibits the CDK9/Cyclin T complex.
Two phase I clinical trials of seliciclib in advanced malignancy have been published [87,88]. Although in one study, dose-limiting toxicities (DLTs) were seen at 800 mg b.i.d., in the other the use of alternative dosing schedules permitted doses as high as 1600 mg twice daily for 3 days every two weeks. Interestingly, the high-grade toxicities were similar, consisting of hyponatremia and hypokalemia, and in each study there was a single case of reversible acute kidney injury. Of 77 total patients, there was one partial response (hepatocellular carcinoma) and 14 patients experienced stabilization of disease. Despite the early promising results in clinical and preclinical studies, however, two phase II trials of seliciclib in solid tumors have been terminated and future development remains uncertain.
SNS-032 (formerly BMS-387032) selectively inhibits CDK2, CDK7, and CDK9, with far less activity against CDK1 and CDK4, as well as a panel of other kinases [89,90]. As with other inhibitors of CDK7 and CDK9, the mechanism of action appears to be down-regulation of short-lived members of the Bcl-2 family including Mcl-1 and XIAP, which is of particular interest in CLL as it has been shown that CLL cells are dependent on consistent expression of antiapoptotic signals to prevent cell death [91–93]. SNS-032 has completed phase I trials in solid tumors and hematologic malignancies [94]. In a trial with 37 patients (19 with CLL), there was limited evidence of efficacy, although this may have been limited by infusion duration or a schedule that was modeled after tumor cell lines as opposed to primary CLL cells. As with flavopiridol, tumor lysis syndrome was the dose-limiting toxicity (DLT) in CLL patients, and other grade 3/4 toxicities were almost entirely hematologic.
TG02 (SB1317) is an orally bioavailable multi-kinase inhibitor which inhibits CDK1, 2, 7, and 9 as well as FLT3, JAK2 in an equipotent fashion [95,96]. TG02 also inhibits other kinases, including ERK5, with lesser potency. It is hoped that this multi-kinase activity will provide benefit beyond less broad inhibitors (e.g. sunitinib), and preclinical studies have shown excellent activity in acute myeloid leukemia blasts [96,97]. It is currently in phase I trials for ALL, AML, CLL, myeloma, and myelodysplastic syndrome [98].
Concluding remarks and future directions
The cyclin dependent kinase inhibitors flavopiridol and dinaciclib have shown remarkable promise in vitro as well as in early phase human trials, both alone and in combination with other agents. As a class, CDK inhibitors all seem to induce apoptosis of B lymphocytes, but misleadingly not necessarily in a way related to the cell-cycle from which they derive their name. As we have described here, the differential efficacy and toxicity profile of the agents in this class likely stems from each agent’s unique combination of inhibition of protein kinases, and CDKs in particular.
As single agents, CDK inhibitors are unlikely to produce prolonged remissions, but their side effect of rapid tumor de-bulking may find utility in bridging otherwise refractory patients to stem-cell transplantation or other therapy. Ultimately, a better understanding of mechanisms of action on a cellular basis will provide a framework for rational combination of targeted therapies in future clinical trials. As with trials of other agents, both patients and science will benefit when eligibility for trials is determined according to the molecular mechanisms of each individual’s tumor, rather than a broad disease grouping. Further, future clinical trials have the opportunity to explore alternative dose plans (for example, stepped-up dosing to ameliorate the effects of tumor lysis) and infusion schedules (in the case of dinaciclib, the anti Mcl-1 effect has been shown to disappear by 24 hours in malignant lymphoid blasts [99]). Alternatively, different delivery vehicles have the potential to prolong the exposure of tumor cells to the active agent: a liposomal formulation of flavopiridol has been developed with the goal of increased half-life, AUC, and consequently efficacy [100]. In addition to combinations of CDK inhibitors with traditional cytotoxic agents, future trials should explore rational combinations of therapy suggested by our ever-better understanding of cell signaling, mRNA transcription, and the specific inhibition patterns of individual agents. Finally, because even as recently as 2012 an entirely new mechanism of action for flavopiridol has been discovered [41], we must remain attuned to the possibility that we will question existing mechanisms or discover new ones in this remarkable and promising class.
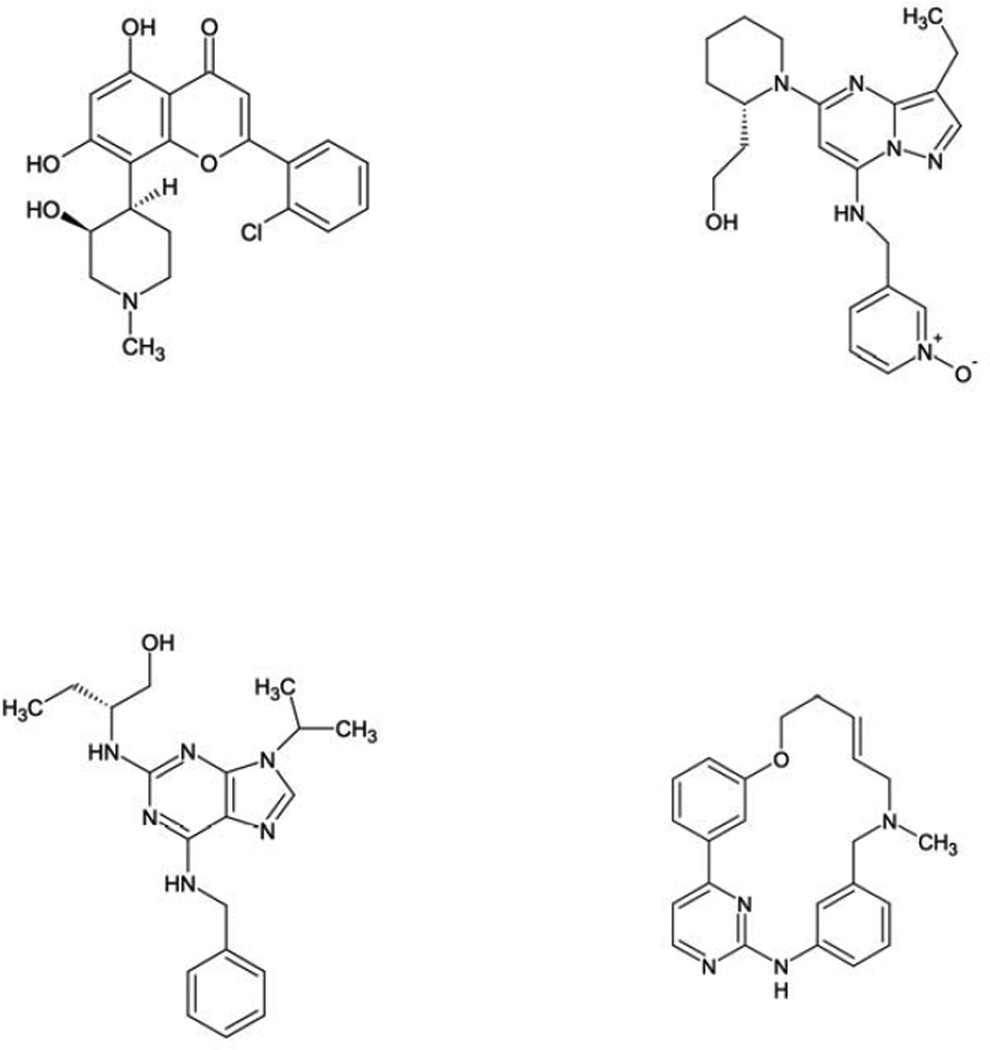
Figure 1.
Molecular structures of representative cyclin-dependent kinase inhibitors alvocidib (flavopiridol; A), dinaciclib (B), seliciclib (C), and TG02 (D).
Table 3.
Quick Profile: Alvocidib (Flavopiridol)
| Drug name | Alvocidib |
|---|---|
| Company | Sanofi-Aventis, Paris, France |
| Other names | Flavopiridol, HMR-1275, L86-8275, NSC-649890 |
| MoA | Inhibition of CDKs 1, 2, 4, 6, 7, and 9 |
| MoR | Autophagy |
| MTD | 80 mg/m2 when given by bolus plus infusion 78 mg/m2/day when given as 72-hour infusion |
| DLT | Acute tumor lysis, cytokine release syndrome, and diarrhea |
| Schedule | Alvocidib days 1, 8, 15 on 28-day cycle Cycle 1: 30 mg/m2 by 30-minute IV bolus, followed by 30 mg/m2 by 4-hour CIVI If no TLS, escalate to Cycle 2: 30 mg/m2 by 30-minute IV bolus, followed by 50 mg/m2 by 4-hour CIVI |
| Plasma concentration | Target > 1.5 µmol/L |
| Plasma half-life | 16 +/− 13.1 h for 60 mg/m2 dose 13.2 +/− 8.8 h for 80 mg/m2 dose |
| Cost | Not yet available outside of trial; this medication is available through CTEP in the United States |
| Other targets | Also inhibits GSK3β at higher nanomolar concentration |
Table 4.
Quick Profile: Dinaciclib
| Drug name | Dinaciclib |
|---|---|
| Company | Merck, Whitehouse Station, NJ |
| Other names | MK-7965, SCH-727965 |
| MoA | Inhibition of CDKs 1, 2, 5, and 9 |
| MoR | Unknown |
| MTD | 12–14 mg/m2 by 2-hour infusion weekly 50 mg/m2 by 2-hour infusion every 21 days 7.4, 10.4 mg/m2 by 8- or 24-hour infusion, respectively |
| DLT | Acute tumor lysis, cytokine release syndrome, and diarrhea |
| Schedule | NHL and CLL: 12–14 mg/m2 by 2-hour infusion on days 1, 8, 15 of 28-day cycle |
| Plasma concentration | 1210 ng/mL after a 29.6 mg/m2 dose |
| Plasma half-life | 3 hours after a 29.6 mg/m2 dose |
| Cost | Not yet available outside of trial; this medication is available through CTEP in the United States |
| Other | Has now entered phase III trials |
Abbreviations: CTEP, Cancer Therapy Evaluation Program; MoA, Mechanism of action; MoR, Mechanism of resistance; MTD, Maximum tolerated dose; DLT, Dose limiting toxicity.
Acknowledgments
This work was supported by Specialized Center of Research from the Leukemia and Lymphoma Society, K12 CA133250, P50 CA140158 and P01 CA81534 from the National Cancer Institute and The D. Warren Brown Foundation.
References
- 1.Hirama T, Koeffler H. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995;86:841–854. [PubMed] [Google Scholar]
- 2.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Jeffrey PD, Russo AA, Polyak K, et al. Mechanism of CDK Activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 5.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56:2973–2978. [PubMed] [Google Scholar]
- 6.Leoncini L, Bellan C, De Falco G. Retinoblastoma gene family expression in lymphoid tissues. Oncogene. 2006;25:5309–5314. doi: 10.1038/sj.onc.1209619. [DOI] [PubMed] [Google Scholar]
- 7.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 8.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 9.Hu B, Mitra J, van den Heuvel S, Enders GH. S and G2 phase roles for CDK2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol Cell Biol. 2001;21:2755–2766. doi: 10.1128/MCB.21.8.2755-2766.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai D, Latham VM, Jr, Zhang X, Shapiro GI. Combined depletion of cell cycle and transcriptional cyclin-dependent kinase activities induces apoptosis in cancer cells. Cancer Res. 2006;66:9270–9280. doi: 10.1158/0008-5472.CAN-06-1758. [DOI] [PubMed] [Google Scholar]
- 11.Wei F-Y, Tomizawa K. Cyclin-dependent kinase 5 (Cdk5): a potential therapeutic target for the treatment of neurodegenerative diseases and diabetes mellitus. Mini Rev Med Chem. 2007;7:1070–1074. doi: 10.2174/138955707782110114. [DOI] [PubMed] [Google Scholar]
- 12.Bregman DB, Pestell RG, Kidd VJ. Cell cycle regulation and RNA polymerase II. Front Biosci. 2000;5:D244–D257. doi: 10.2741/bregman. [DOI] [PubMed] [Google Scholar]
- 13.Oelgeschläger T. Regulation of RNA polymerase II activity by CTD phosphorylation and cell cycle control. J Cell Physiol. 2002;190:160–169. doi: 10.1002/jcp.10058. [DOI] [PubMed] [Google Scholar]
- 14.Sims RJ, Belotserkovskaya R, Reinberg D. Elongation by RNA polymerase II: the short and long of it. Genes & Development. 2004;18:2437–2468. doi: 10.1101/gad.1235904. [DOI] [PubMed] [Google Scholar]
- 15.Kitada S, Andersen J, Akar S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood. 1998;91:3379–3389. [PubMed] [Google Scholar]
- 16.Reed JC. Molecular biology of chronic lymphocytic leukemia. Semin Oncol. 1998;25:11–18. [PubMed] [Google Scholar]
- 17.Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-xL is an essential survival protein of human myeloma cells. Blood. 2002;100:194–199. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 18.Pepper C, Thomas A, Hoy T, Cotter F, Bentley P. Antisense-mediated suppression of Bcl-2 highlights its pivotal role in failed apoptosis in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;107:611–615. doi: 10.1046/j.1365-2141.1999.01726.x. [DOI] [PubMed] [Google Scholar]
- 19.Chawla-Sarkar M, Bae SI, Reu FJ, et al. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–923. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 20.Manohar SM, Rathos MJ, Sonawane V, Rao SV, Joshi KS. Cyclin-dependent kinase inhibitor, P276-00 induces apoptosis in multiple myeloma cells by inhibition of Cdk9-T1 and RNA polymerase II-dependent transcription. Leuk Res. 2011;35:821–830. doi: 10.1016/j.leukres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Mohanakumara P, Sreejayan N, Priti V, et al. Dysoxylum binectariferum Hook.f (Meliaceae), a rich source of rohitukine. Fitoterapia. 2010;81:145–148. doi: 10.1016/j.fitote.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Kaur G, Stetler-Stevenson M, Sebers S, et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J Natl Cancer Inst. 1992;84:1736–1740. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- 23.Bible KC, Kaufmann SH. Flavopiridol: a cytotoxic flavone that induces cell death in noncycling A549 human lung carcinoma cells. Cancer Res. 1996;56:4856–4861. [PubMed] [Google Scholar]
- 24.Brüsselbach S, Nettelbeck DM, Sedlacek HH, Müller R. Cell cycle-independent induction of apoptosis by the anti-tumor drug Flavopiridol in endothelial cells. Int J Cancer. 1998;77:146–152. doi: 10.1002/(sici)1097-0215(19980703)77:1<146::aid-ijc22>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst. 2000;92:376–387. doi: 10.1093/jnci/92.5.376. [DOI] [PubMed] [Google Scholar]
- 26.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 27.de Azevedo WF, Jr, Canduri F, da Silveira NJF. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem Biophys Res Commun. 2002;293:566–571. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 28.Baumli S, Lolli G, Lowe ED, et al. The structure of P-TEFb (CDK9/cyclin T1), its complex with flavopiridol and regulation by phosphorylation. EMBO J. 2008;27:1907–1918. doi: 10.1038/emboj.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.König A, Schwartz GK, Mohammad RM, Al-Katib A, Gabrilove JL. The novel cyclin-dependent kinase inhibitor flavopiridol downregulates Bcl-2 and induces growth arrest and apoptosis in chronic B-cell leukemia lines. Blood. 1997;90:4307–4312. [PubMed] [Google Scholar]
- 30.Parker BW, Kaur G, Nieves-Neira W, et al. Early induction of apoptosis in hematopoietic cell lines after exposure to flavopiridol. Blood. 1998;91:458–465. [PubMed] [Google Scholar]
- 31.Arguello F, Alexander M, Sterry JA, et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity In vivo against human leukemia and lymphoma xenografts. Blood. 1998;91:2482–2490. [PubMed] [Google Scholar]
- 32.Byrd JC, Shinn C, Waselenko JK, et al. Flavopiridol Induces Apoptosis in Chronic Lymphocytic Leukemia Cells Via Activation of Caspase-3 Without Evidence of bcl-2 Modulation or Dependence on Functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 33.Christian BA, Grever MR, Byrd JC, Lin TS. Flavopiridol in chronic lymphocytic leukemia: a concise review. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S179–185. doi: 10.3816/CLM.2009.s.009. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro GI, Koestner DA, Matranga CB, Rollins BJ. Flavopiridol induces cell cycle arrest and p53-independent apoptosis in non-small cell lung cancer cell lines. Clin Cancer Res. 1999;5:2925–2938. [PubMed] [Google Scholar]
- 35.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–397. [PubMed] [Google Scholar]
- 36.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grever MR, Lucas DM, Dewald GW, et al. Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: results from the US intergroup phase III trial E2997. J Clin Oncol. 2007;25:799–804. doi: 10.1200/JCO.2006.08.3089. [DOI] [PubMed] [Google Scholar]
- 38.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. The Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen R, Keating MJ, Gandhi V, Plunkett W. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106:2513–2519. doi: 10.1182/blood-2005-04-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain S-RA, Lucas DM, Johnson AJ, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111:3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahoney E, Lucas DM, Gupta SV, et al. ER stress and autophagy: new discoveries in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Blood. 2012;120:1262–1273. doi: 10.1182/blood-2011-12-400184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senderowicz AM, Headlee D, Stinson SF, et al. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol. 1998;16:2986–2999. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro GI, Supko JG, Patterson A, et al. A phase II trial of the cyclin-dependent kinase inhibitor flavopiridol in patients with previously untreated stage IV non-small cell lung cancer. Clin Cancer Res. 2001;7:1590–1599. [PubMed] [Google Scholar]
- 44.Stadler WM, Vogelzang NJ, Amato R, et al. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in metastatic renal cancer: a University of Chicago Phase II Consortium study. J Clin Oncol. 2000;18:371–375. doi: 10.1200/JCO.2000.18.2.371. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz GK, O’Reilly E, Ilson D, et al. Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. J Clin Oncol. 2002;20:2157–2170. doi: 10.1200/JCO.2002.08.080. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz GK, Ilson D, Saltz L, et al. Phase II study of the cyclin-dependent kinase inhibitor flavopiridol administered to patients with advanced gastric carcinoma. J Clin Oncol. 2001;19:1985–1992. doi: 10.1200/JCO.2001.19.7.1985. [DOI] [PubMed] [Google Scholar]
- 47.Lin TS, Howard OM, Neuberg DS, Kim HH, Shipp MA. Seventy-two hour continuous infusion flavopiridol in relapsed and refractory mantle cell lymphoma. Leuk Lymphoma. 2002;43:793–797. doi: 10.1080/10428190290016908. [DOI] [PubMed] [Google Scholar]
- 48.Flinn IW, Byrd JC, Bartlett N, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res. 2005;29:1253–1257. doi: 10.1016/j.leukres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Byrd JC, Peterson BL, Gabrilove J, et al. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19805. Clin Cancer Res. 2005;11:4176–4181. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 50.Shinn C, Larsen D, Suarez J-R. Flavopiridol sensitivity of chronic lymphocytic leukemia (CLL) cells in vitro varies based upon species specific drug protein binding. Blood. 2000;96:294. [Google Scholar]
- 51.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Messmann RA, Ullmann CD, Lahusen T, et al. Flavopiridol-related proinflammatory syndrome is associated with induction of interleukin-6. Clin Cancer Res. 2003;9:562–570. [PubMed] [Google Scholar]
- 53.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephens DM, Ruppert AS, Blum K, et al. Flavopiridol treatment of patients aged 70 or older with refractory or relapsed chronic lymphocytic leukemia is a feasible and active therapeutic approach. Haematologica. 2012;97:423–427. doi: 10.3324/haematol.2011.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 56.Lanasa MC, Andritsos L, Brown JR, et al. Interim analysis of EFC6663, a multicenter phase 2 study of alvocidib (flavopiridol), demonstrates clinical responses among patients with fludarabine refractory CLL. Blood. 2010;116(Suppl.:Abstract):58. [Google Scholar]
- 57.Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu-Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120:3986–3996. doi: 10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrajoli A, Lee B-N, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene. 2003;22:7108–7122. doi: 10.1038/sj.onc.1206863. [DOI] [PubMed] [Google Scholar]
- 61.Dai Y, Rahmani M, Pei X-Y, Dent P, Grant S. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104:509–518. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- 62.Holkova B, Perkins EB, Ramakrishnan V, et al. Phase I trial of bortezomib (PS-341; NSC 681239) and alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory B-cell neoplasms. Clin Cancer Res. 2011;17:3388–3397. doi: 10.1158/1078-0432.CCR-10-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paruch K, Dwyer MP, Alvarez C, et al. Discovery of dinaciclib (SCH 727965): a potent and selective inhibitor of cyclin-dependent kinases. ACS Med Chem Lett. 2010;1:204–208. doi: 10.1021/ml100051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parry D, Guzi T, Shanahan F, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Molecular Cancer Therapeutics. 2010;9:2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 65.Johnson AJ, Yeh Y-Y, Smith LL, et al. The novel cyclin-dependent kinase inhibitor dinaciclib (SCH727965) promotes apoptosis and abrogates microenvironmental cytokine protection in chronic lymphocytic leukemia cells. Leukemia. 2012;[Epub ahead of print] doi: 10.1038/leu.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baiocchi RA, Flynn JM, Jones JA, et al. Early evidence of anti-lymphoma activity of the cyclin dependent kinase inhibitor dinaciclib (SCH 727965) in heavily pre-treated low grade lymphoma and diffuse large cell lymphoma patients. Blood. 2010;116 (Suppl.):Abstract:3966. [Google Scholar]
- 67.Flynn JM, Jones JA, Andritsos L, et al. Update on the phase I study of the cyclin dependent kinase inhibitor dinaciclib (SCH 727965) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): confirmation of clinical activity and feasibility of long-term administration. Blood. 2010;116 (Suppl.):Abstract:1396. [Google Scholar]
- 68.Mita MM, Mita AC, Mosely J, et al. A phase I study of the CDK inhibitor dinaciclib (SCH 727965) administered every 3 weeks in patients (pts) with advanced malignancies: Final results. J Clin Oncol. 2011;29 (Suppl.):Abstract:3080. [Google Scholar]
- 69.Flynn JM, Jones JA, Andritsos L, et al. Phase I study of the CDK inhibitor dinaciclib (SCH 727965) in patients (pts) with relapsed/refractory CLL. J Clin Oncol. 2011;29 (Suppl.):Abstract:6623. [Google Scholar]
- 70.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siemeister G, Lücking U, Wengner AM, et al. BAY 1000394, a novel cyclin-dependent kinase inhibitor, with potent antitumor activity in mono- and in combination treatment upon oral application. Mol Cancer Ther. 2012;11:2265–2273. doi: 10.1158/1535-7163.MCT-12-0286. [DOI] [PubMed] [Google Scholar]
- 72.Joshi KS, Rathos MJ, Joshi RD, et al. In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00. Mol Cancer Ther. 2007;6:918–925. doi: 10.1158/1535-7163.MCT-06-0613. [DOI] [PubMed] [Google Scholar]
- 73.Joshi KS, Rathos MJ, Mahajan P, et al. P276-00, a novel cyclin-dependent inhibitor induces G1-G2 arrest, shows antitumor activity on cisplatin-resistant cells and significant in vivo efficacy in tumor models. Mol Cancer Ther. 2007;6:926–934. doi: 10.1158/1535-7163.MCT-06-0614. [DOI] [PubMed] [Google Scholar]
- 74.Raje N, Hideshima T, Mukherjee S, et al. Preclinical activity of P276-00, a novel small-molecule cyclin-dependent kinase inhibitor in the therapy of multiple myeloma. Leukemia. 2009;23:961–970. doi: 10.1038/leu.2008.378. [DOI] [PubMed] [Google Scholar]
- 75.Hirte HW, Raghunadharao D, Baetz T, et al. A phase 1 study of the selective cyclin dependent kinase inhibitor P276-00 in patients with advanced refractory neoplasms. J Clin Oncol. 2007;25:14117. [Google Scholar]
- 76.Chu X-J, DePinto W, Bartkovitz D, et al. Discovery of [4-Amino-2-(1-methanesulfonylpiperidin-4-ylamino)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone (R547), a potent and selective cyclin-dependent kinase inhibitor with significant in vivo antitumor activity. J Med Chem. 2006;49:6549–6560. doi: 10.1021/jm0606138. [DOI] [PubMed] [Google Scholar]
- 77.DePinto W, Chu X-J, Yin X, et al. In vitro and in vivo activity of R547: a potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol Cancer Ther. 2006;5:2644–2658. doi: 10.1158/1535-7163.MCT-06-0355. [DOI] [PubMed] [Google Scholar]
- 78.Diab S, Eckhardt S, Tan A, et al. A phase I study of R547, a novel, selective inhibitor of cell cycle and transcriptional cyclin dependent kinases (CDKs) J Clin Oncol. 2007;25 (Suppl.):Abstract:3528. [Google Scholar]
- 79.Veselý J, Havlicek L, Strnad M, et al. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 80.De Azevedo WF, Leclerc S, Meijer L, et al. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human Cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 81.Meijer L, Borgne A, Mulner O, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases Cdc2, Cdk2 and Cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 82.McClue SJ, Blake D, Clarke R, et al. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine) Int J Cancer. 2002;102:463–468. doi: 10.1002/ijc.10738. [DOI] [PubMed] [Google Scholar]
- 83.Mgbonyebi OP, Russo J, Russo IH. Roscovitine induces cell death and morphological changes indicative of apoptosis in MDA-MB-231 breast cancer cells. Cancer Res. 1999;59:1903–1910. [PubMed] [Google Scholar]
- 84.Edamatsu H, Gau CL, Nemoto T, Guo L, Tamanoi F. CDK inhibitors, roscovitine and olomoucine, synergize with farnesyltransferase inhibitor (FTI) to induce efficient apoptosis of human cancer cell lines. Oncogene. 2000;19:359–3068. doi: 10.1038/sj.onc.1203625. [DOI] [PubMed] [Google Scholar]
- 85.Hahntow IN, Schneller F, Oelsner M, et al. Cyclin-dependent kinase inhibitor roscovitine induces apoptosis in chronic lymphocytic leukemia cells. Leukemia. 2004;18:747–755. doi: 10.1038/sj.leu.2403295. [DOI] [PubMed] [Google Scholar]
- 86.Alvi AJ, Austen B, Weston VJ, et al. A novel CDK inhibitor, CYC202 (R-roscovitine), overcomes the defect in p53-dependent apoptosis in B-CLL by down-regulation of genes involved in transcription regulation and survival. Blood. 2005;105:4484–4491. doi: 10.1182/blood-2004-07-2713. [DOI] [PubMed] [Google Scholar]
- 87.Benson C, White J, De Bono J, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le Tourneau C, Faivre S, Laurence V. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer. 2010;46:3243–3250. doi: 10.1016/j.ejca.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Misra RN, Xiao H, Kim KS. N-(cycloalkylamino) acyl-2-aminothiazole inhibitors of cyclin-dependent kinase 2 etal N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide (BMS-387032), a highly efficacious and selective antitumor agent. J Med Chem. 2004;47:1719–1728. doi: 10.1021/jm0305568. [DOI] [PubMed] [Google Scholar]
- 90.Chen R, Wierda WG, Chubb S, et al. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2009;113:4637–4645. doi: 10.1182/blood-2008-12-190256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic Bcl-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 92.Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 93.Del Gaizo Moore V, Brown JR, Certo M, et al. Chronic lymphocytic leukemia requires Bcl2 to sequester prodeath Bim, explaining sensitivity to Bcl2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tong W-G, Chen R, Plunkett W, et al. Phase I and pharmacologic study of SNS-032, a potent and selective Cdk2, 7, and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia and multiple myeloma. J Clin Oncol. 2010;28:3015–3022. doi: 10.1200/JCO.2009.26.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burrows F, Goh K, Novotny-Diermayr V, et al. A novel, multi-kinase inhibitor with potent activity against solid tumors. J Clin Oncol. 2010;28 (Suppl.):Abstract:e13549. [Google Scholar]
- 96.Goh KC, Novotny-Diermayr V, Hart S, et al. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia. 2012;26:236–243. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- 97.Pallis M, Abdul-Aziz A, Burrows F, et al. The multi-kinase inhibitor TG02 overcomes signalling activation by survival factors to deplete MCL1 and XIAP and induce cell death in primary acute myeloid leukaemia cells. Br J Haematol. 2012;159:191–203. doi: 10.1111/bjh.12018. [DOI] [PubMed] [Google Scholar]
- 98.Roboz GJ, Khoury HJ, Shammo JM, et al. Phase I dose escalation study of TG02 in patients with advanced hematologic malignancies. J Clin Oncol. 2012;30 (Suppl.):Abstract:6577. [Google Scholar]
- 99.Gojo I, Walker A, Cooper M, et al. Phase II study of the cyclin-dependent kinase (CDK) inhibitor dinaciclib (SCH 727965) in patients with advanced acute leukemias. Blood. 2010;116 (Suppl.):Abstract:3287. [Google Scholar]
- 100.Yang X, Zhao X, Phelps MA, et al. A novel liposomal formulation of flavopiridol. Int J Pharm. 2009;365:170–174. doi: 10.1016/j.ijpharm.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]