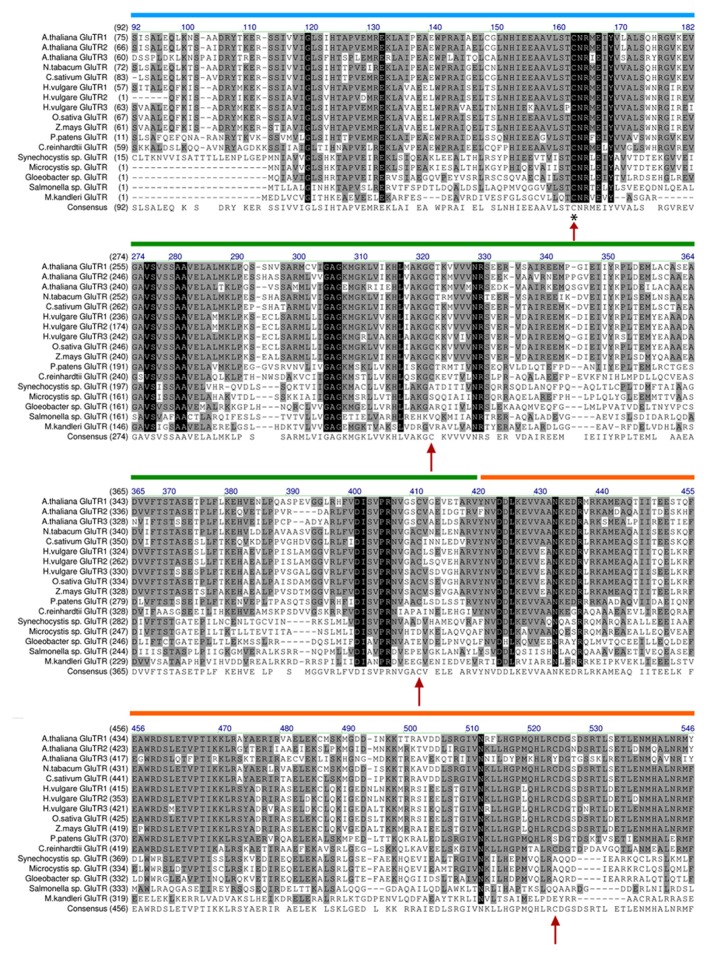
FIGURE 3.
Alignment of GluTR sequences of selected plant and bacterial species. Arrows denote conserved cysteine residues in the GluTR sequence. The cysteine marked with an asterisk (*) was found to perform the nucleophilic attack on tRNAGLU and is thus involved in the catalytic reaction of GluTR (see Moser et al., 1999/therein: Cys48 here: Cys163). Interestingly, only Cys163 is conserved in all analyzed GluTR amino acid sequences. The other cysteines (Cys322, Cys411, and Cys524) are only conserved in GluTR of higher plants (with the exception of HEMA3 encoded GLUTR). The alignment is depicted without the proposed transit peptide of plant GluTRs and highlights the sequence harboring the conserved cysteines. Colored bars: domains annotated by Schubert et al. (2002). Blue: catalytic domain; green: NADPH-binding domain; orange: dimerization domain.