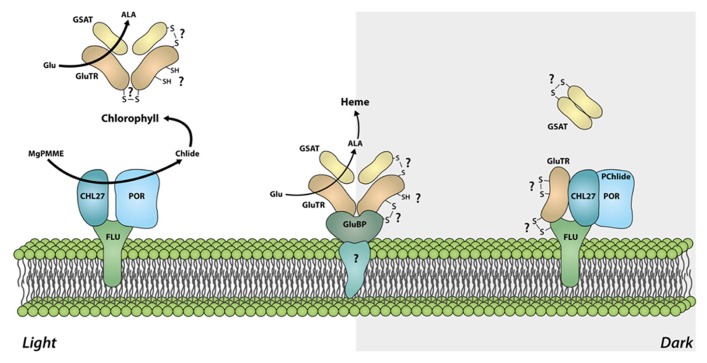
FIGURE 4.
Model of the redox-dependent organization of the ALA synthesizing enzyme complexes. In light, the tetrapyrrole biosynthetic pathway is actively channeling substrates from GluTR to GSAT and ensures high activity of the ALA synthesizing enzymes and allocation of sufficient amounts of the precursors for the synthesis of different end products. In darkness, GluTR interacts with FLU, which is assembled with CHL27 and POR. This protein complex represses ALA synthesis. It is assumed that the reduction of target cysteine residues in intramolecular disulfide bonds of GluTR are the pre-condition for the formation of a heterotetrameric GluTR2GSAT2 complex or for high activity of ALA synthesizing enzymes in light. Oxidation of cysteine residues or formation of intra-/intermolecular disulfide bonds in darkness could additionally participate in the deactivation of GluTR (e.g., by stabilizing the interaction with FLU) and thus mediating the dark repression of ALA synthesis. Spatial separation of GluTR for heme synthesis by the GluTR-binding protein (GluBP) ensures ALA synthesis for the heme-synthesizing branch, although the bulk of ALA synthesizing activity is repressed by FLU.