Table 1.
Optimization of typical reaction conditions.a
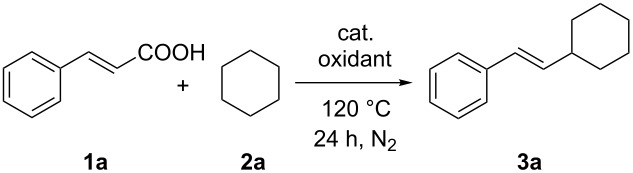 | |||
| Entry | cat. (mol %) | oxidant | yield (%)b |
| 1 | FeCl2·4H2O (20) | DTBP | 54 |
| 2 | FeCl2·4H2O (20) | TBHP | 38 |
| 3 | FeCl2·4H2O (20) | DTBP | 68c |
| 4 | FeCl3 (20) | DTBP | Trace |
| 5 | Ferrocene (20) | DTBP | 74 |
| 6 | Fe2O3 (20) | DTBP | 78 |
| 7 | Fe3O4 (20) | DTBP | 80 |
| 8 | Fe(acac)3 (20) | DTBP | 91 |
| 9 | Fe(acac)3 (20) | K2S2O8 | N.D. |
| 10 | Fe(acac)3 (20) | H2O2d | 21 |
| 11 | Fe(acac)3 (20) | TBPB | 49 |
| 12 | Fe(acac)3 (10) | DTBPe | 63 |
| 13f | Fe(acac)3 (20) | DTBP | 69 |
| 14 | Fe(acac)3 (20)g | DTBP | 79 |
| 15 | Fe(acac)3 (20) | – | N.D. |
| 16 | – | DTBP | N.D. |
aCatalytic conditions: cinnamic acid (0.3 mmol), cyclohexane (2 mL), iron catalyst (20 mol %), oxidant (2.0 equiv), 120 °C, 24 h, N2. bIsolated yields based on cinnamic acid. cUsing 1,10-phenanthroline (30 mol %) as the ligand. d30% aqueous solution. e5 equiv. f12 h. g110 °C.
