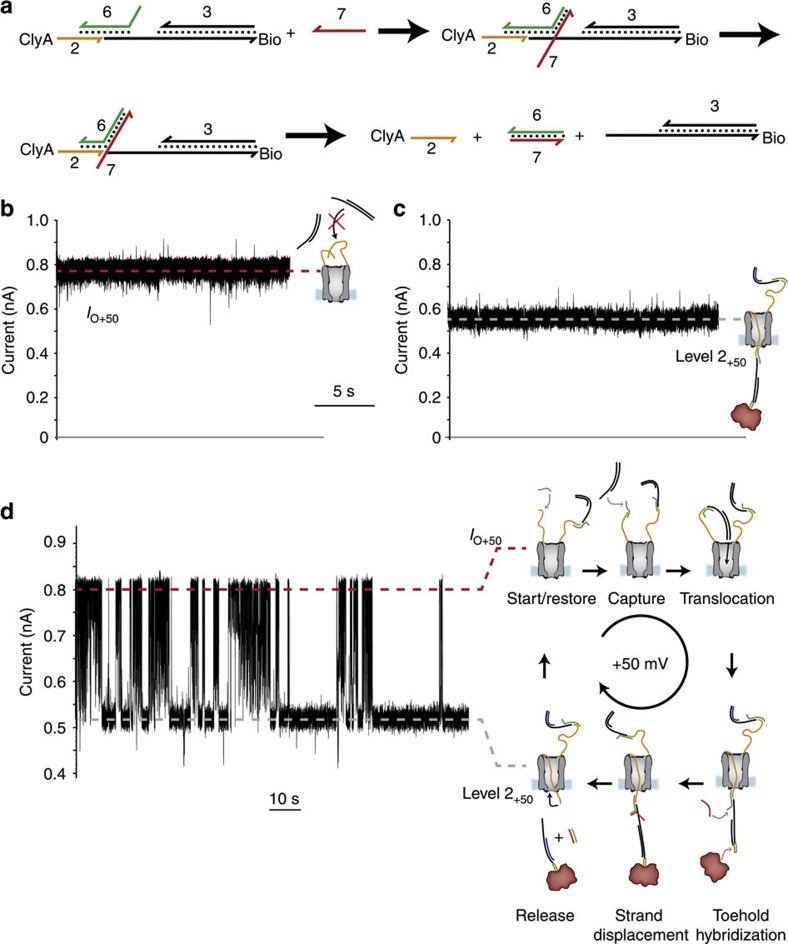
Figure 4. Transport of DNA through ClyA nanopores.
(a) Schematic representation of the strand-displacement reaction that promoted the release of DNA from the pore. The suffix bio indicates a biotin moiety. (b) At +50 mV and in the presence of 3 (0.3 μM), ClyA-2 showed a steady open pore current (IO+50=0.85±0.01 nA, mean±s.d., n=3), showing that the ssDNA strands attached to the pore did not thread through the lumen of the pore and prevented the translocation of dsDNA form solution. (c) The addition of the ssDNA strand 6 (40 nM) to the cis chamber in the presence of the strand 3 and 0.3 μM neutravidin (tetramer) in the trans compartment produced long-lasting current blockades with IRES+50=0.70±0.02 (mean±s.d., level 2+50=0.59±0.02 nA, mean±s.d., n=5), indicating that the dsDNA hybrid threaded the pore. (d) The subsequent addition of 1 μM of 7 to the trans chamber (+50 mV) promoted the release of the DNA thread by a strand displacement reaction. 2 returned to the cis compartment against the applied potential and the open pore current was restored. Specific dsDNA molecules were then sequentially captured and released as shown by successive blocked and open pore currents. In this experiment, the applied potential was set to +50 mV and not to +100 mV as in the rest of this work. This is because at +100 mV ClyA-2 occasionally produced long current blockades that were similar to typical events provoked by the capture of non-specific DNA (Supplementary Fig. S5c). Such current blockades were not observed at lower applied potentials; hence, we performed this experiment at +50 mV. In addition, at+50 mV the rate of DNA capture was lower than at +100 mV, and therefore the cycles of capture and release were more easily observed. The electrical recordings were carried out in 2.5 M NaCl, 15 mM Tris HCl, pH 7.5 at 22 °C. Data were recorded by applying a 2-kHz low-pass Bessel filter and using a 100μs (10 kHz) sampling rate.