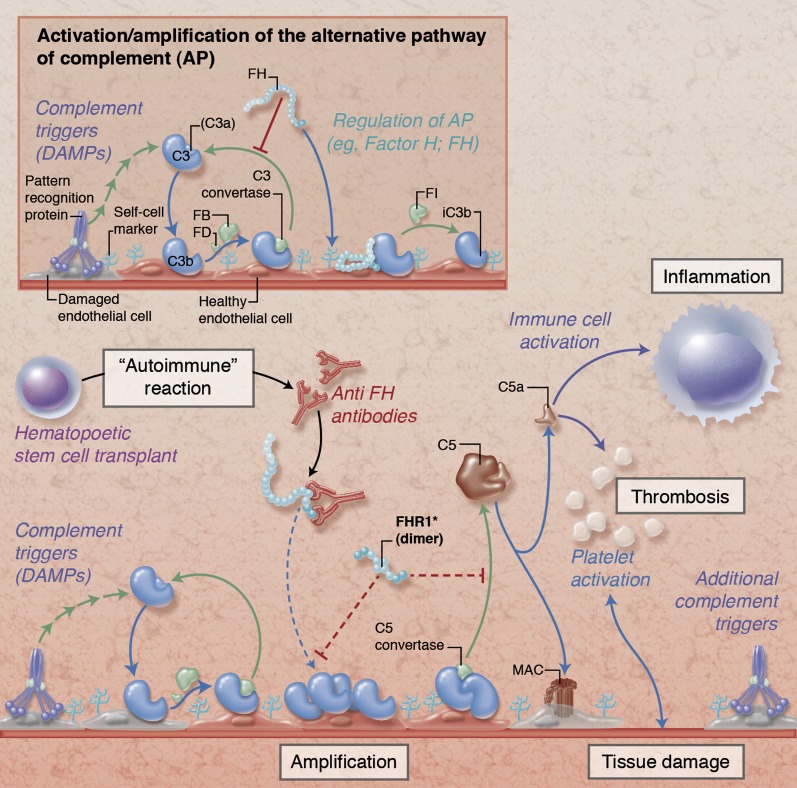
Simplified illustration of complement activation and regulation on damaged endothelial cells. Under physiological conditions (insert), initial tissue injury with exposure of damage-associated molecular patterns (DAMPs) that trigger complement activation leads to opsonization with C3b and formation of C3 convertases that amplify the response by generating additional C3b. Together with membrane regulators of complement (not shown), circulating FH protects host cells by binding to cell surface components (eg, glycosaminoglycans), promoting decay of the convertases and degrading C3b via factor I (FI). In HSCT-associated TMA (main figure), these regulatory mechanisms are potentially impaired by autoantibodies against FH (anti-FH) and/or deletions in genes encoding FH-related protein 1 (FHR1). While the exact pathophysiological involvement of anti-FH and FHR1 remains to be explored, the expected resulting dysregulation facilitates amplification of the complement response with subsequent cleavage of C5 and generation of the anaphylatoxin C5a and the membrane attack complex (MAC). These effectors contribute to thromboinflammatory consequences by activating immune cells, endothelium, and platelets and by affecting membrane integrity, thereby fueling a vicious cycle of tissue damage that fosters additional complement activation. Proposed physiological mechanisms of FHR1 are indicated with dotted red lines in the main figure; the functional implication of FHR deletions in HSCT-associated TMA are not yet known. Professional illustration by Alice Y. Chen.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.