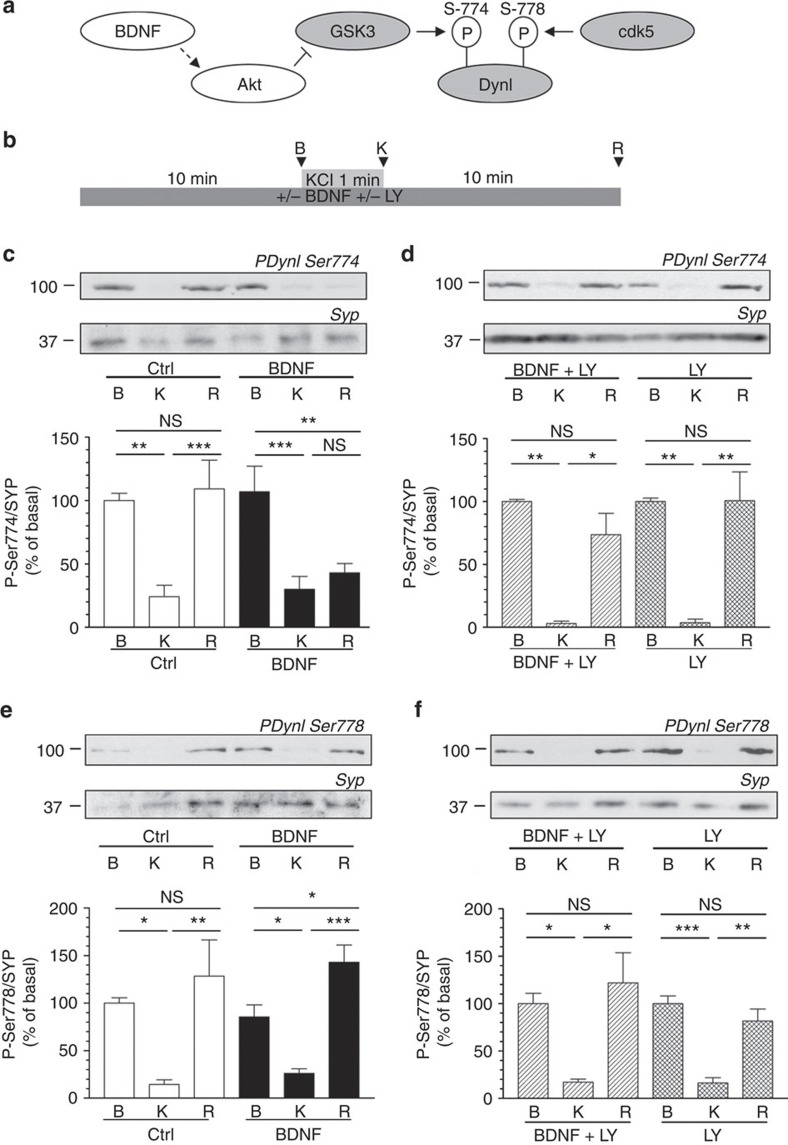
Figure 2. BDNF inhibits dynamin I rephosphorylation on Ser-774 via a PI3K-dependent cascade.
(a) Scheme illustrating that cdk5 phosphorylates Ser-778 on dynamin I (DynI), allowing GSK3 to phosphorylate Ser-774. Our hypothesis was that BDNF would activate Akt to phosphorylate and inactivate GSK3, thus inhibiting its ability to rephosphorylate Ser-774 on DynI. (b) CGNs were placed in incubation medium for 10 min before stimulation with 50 mM KCl (1 min). Following stimulation, CGNs were repolarized for 10 min. Samples were prepared from cultures before stimulation (basal, B), directly after KCl stimulation (K) or after 10 min repolarization (R) as indicated by arrowheads. BDNF (100 ng ml−1) or LY294002 (LY, 10 μM) were present throughout the experiment where indicated. Lysates were separated by SDS–PAGE and probed for either (c,d) phospho-Ser-774 (PDynI Ser-774) or (e,f) phospho-Ser-778 (PDynI Ser-778) on dynamin I on immunoblots. Quantitative analysis is shown in the graphs in (c–f). These graphs display the extent of phosphorylation of either Ser-774 (c,d) or Ser-778 (e,f). All values were normalized to the amount of synaptophysin (SYP) as a loading control, and expressed as a percentage of Basal±s.e.m. (c) n=9, (d) n=3, (e) n=5, (f) n=4, one-way analysis of variance (ANOVA). *P<0.05, **P<0.01, ***P<0.001.