Abstract
Background:
Warts are sometimes resistant or they tend to recur after every possible destructive therapy. Immunotherapy with skin-test antigens has been used as a viable therapeutic option in such recalcitrant cases.
Aim:
The aim of the study was to evaluate the response of resistant or recurrent warts to intralesional Candida albicans antigen immunotherapy.
Materials and Methods:
A total of 40 patients with resistant or recurrent warts who showed a positive test reaction to C. albicans antigen were given intralesional injections of purified C. albicans antigen solution in a single wart at 3-weekly intervals for a total of three doses. The patients were monitored for resolution of the injected wart as well as other untreated warts. The patients who responded positively were then followed up for any relapses over the next 6 months. Adverse events, if any, were also documented.
Results:
Of the 40 patients enrolled in the study, 34 completed the total treatment protocol of three injections and 6 months of follow-up. In these 34 patients, 19 (56%) showed a complete resolution of warts at all places on the body. In addition, two patients (6%) showed a partial or complete resolution of the treated wart, but there was no effect on the untreated warts. Thirteenpatients (38%) failed to show any response to the treatment regimen. In all patients showing resolution of all the warts, there were no relapses at any site over the next 6 months of follow-up. The most common adverse effect seen was pain during the intralesional injection.
Conclusions:
Intralesional Candida immunotherapy seems to be an effective treatment option in more than half of the patients who fail to show a positive response to destructive modes of treatment or in whom there are multiple recurrences.
Limitations:
The small sample size and lack of control group are the main limitations of the study.
Keywords: Candida injections, immunotherapy, recurrent veruccae, treatment, veruccae
Introduction
What was known?
Treatment of recurrent or resistant warts is still a very difficult proposition.
Immunotherapy is an exciting treatment option for such cases.
Multiple skin test antigens have been used in immunotherapy of warts.
There are still no clinical studies from India on Candida immunotherapy in the management of warts.
Warts are a common sight in dermatology OPDs and they constitute the commonest cutaneous manifestation of human papilloma virus (HPV) infection. Warts (verrucae) are primarily treated by destructive therapies such as topical keratolytics,[1,2,3] electro fulguration or radiofrequency ablation,[4] liquid nitrogen cryotherapy,[5,6] and laser vaporization with either pulsed dye laser or CO2 laser.[7,8,9] Other options include intralesional chemotherapeutic agents such as bleomycin,[10,11] oral immune modulators like cimetidine,[12,13] levamisole,[14] or even oral antivirals like cidofovir.[15] Response to any of these treatment options is highly variable and patient dependent. Thus, we have patients in whom the warts resolve spontaneously with the simplest treatment or even without any medical treatment, and at the other extreme, there are patients in whom the warts seem to be resistant to every possible mode of treatment. Moreover, even after an individual patient has responded to any of these treatment options, there are chances of relapse and it is quite common to come across patients with multiple troublesome recurrences.
In patients with unresponsive or recurrent warts, immunotherapy is an exciting treatment option and over the last few years, various treatment methods have been described that basically stimulate the immunologic response to HPV. Immunotherapy in warts can be administered by various methods. The first and the simplest method is topical application of certain inorganic molecules that are capable of eliciting a contact hypersensitivity reaction with secondary activation of an immunological response,[16] or even topical applications of immune modulators like imiquimod.[17,18] Another way is to inject the immunotherapeutic agent into the wart tissue itself. In case of multiple warts, it is usually the mother wart or the wart that appeared first that is injected. The possible mechanism of action of immunotherapy is that the injection or application of the immunotherapeutic agent leads to proliferation of HPV-specific mononuclear cells that mediate an immunologic attack against the wart tissue.[19] What is interesting is that this immune attack has a potential to resolvethe distant warts as well and not the wart alone that has been primarily injected.
Studies have been conducted on the effectiveness of immunotherapy with single as well as multiple test antigens in treatment of warts. The first such study documented the efficacy of Candida skin-test antigen in wart resolution.[19] Subsequent studies were conducted on this antigen and also on other antigens such as mumps, trichophyton, and even a combination of multiple test antigens.[20,21,22,23,24,25] All of these studies have shown that immunotherapy not only causes a resolution of the treated wart but also leads to clearance of distant warts, at least in a subset of the responders.
Materials and Methods
This study was conducted from November 2010 to January 2012 at our institute on patients suffering from resistant or recurrent veruccae anywhere on the body. A proper permission was obtained from the local Ethics Committee before the initiation of the study. Inclusion criteria included patients with multiple veruccae vulgaris that were either resistant to treatment or had relapsed at least once after treatment with any of the tissue-destructive modalities. The veruccae were termed as resistant only if they failed to show any response to at least one destructive mode of treatment. Patients with any evidence of immunosuppression including HIV infection, patients with any eczematous skin disorder, those with any history of hypersensitivity to Candida albicans antigen, pregnant or lactating women, patients on immunosuppressant or immune modulatory drugs, and patients younger than 12 years of age were excluded from the study. Patients were informed about all the modalities of treatment available for warts and the relative merits and demerits of C. albicans immunotherapy including a possible non-response to the treatment regimen. An informed consent form was then given to the patients and they were made to sign it themselves or by their guardians. The warts were then photographed and counted and the size of the injected wart was also noted down.
Before inclusion into the study, a test dose of the Candida antigen was given intradermally on the flexor aspect of the forearm and the area of injection was marked with a permanent marker pen. The patient was called after 2 days (48 h) and the reaction at the intradermal injection site was noted down. Erythema and induration at the site were observed, and the response to the test dose was taken as positive if the area of erythema and induration was at least 5 mm in diameter. Only those patients who showed a positive response to the Candida test antigen were enrolled and taken up for treatment with C. albicans antigen injections. A cohort of 40 such patients was thus selected for the study and each of these patients received intralesional injection of purified C. albicans antigen solution into the mother wart, i.e., the earliest or initial wart on the body. If the patient was not sure about the initial wart, then the largest wart was injected irrespective of its temporal association. The Candida test antigen used was the ‘CREDISOL skin test solution’ marketed by Creative Drug Industries, Mumbai, India. Injections were given into the substance of the wart using a 27-guage needle, and a total of three injections were administered at 3-week intervals. The same wart was injected on all the three occasions in every patient. The volume of the test antigen used was 0.1 ml on every occasion irrespective of the severity of response to the initial intradermal test dose. Thus, a total of 0.3 ml of the test antigen was used in each patient. The patients were examined at each follow-up injection and any regression in the size of the warts was noted down. Digital photographs were also repeated at every follow-up visit, and adverse events, if any, were also noted down. Each patient was told about the possibility of a febrile reaction after the injection and was advised to take antipyretics in case of such an eventuality. Patients who showed no or a partial response after three injections were called for a final follow-up 1 month after the date of the final injection for eventual evaluation of the therapeutic result. However, all positive responders were further followed up for a period of 6 months after the treatment, and recurrences, if any, were noted down. In those patients where the shaving area of the face was involved, shaving blades were prohibited during the treatment as well as for the next 12-weeks of follow-up.
Response to treatment was noted down at every follow-up visit with clinical examination of the treated as well as untreated warts along with photographic documentation. The wart was said to have completely resolved only when there was complete disappearance of the hyperkeratosis and thickening of the skin. The wart was considered to have shown a partial resolution if there was 50-75% reduction in the original diameter of the treated wart. Reduction in the size of the treated wart by < 50% was considered to be a non-response.
Patients who did not show any response to the immunotherapeutic regimen were offered alternative treatment options.
Results
Of the 40 patients enrolled for the study, six could not complete the course of three injections as they did not come for further injections after their first dose only. These patients were dropped from the study and thus we had 34 patients available for assessment at the end of the study.
Age of these patients ranged from 14 to 36 years, with a mean of 24.3 years. Male patients were more commonly represented in the study group as there were 20 male and only 14 female patients in the study population.
The duration of warts ranged from a minimum of 3months to a maximum of 8 years, with a mean of 1.2 years.
Warts were seen on more than one site in 19 patients. Sites where the warts were present included the face (22), hands (18), feet or legs (14), and forearms (4). Thus, face was the commonest site to be treated followed by the hands.
Pain at the injection site was the commonest adverse effect reported and this was reported by majority of the patients. The pain occurred at the time of injection only and was the most severe in intensity when the fingers were injected. In fact, all patients in whom the fingers, especially the peri-ungual warts were injected, complained of severe pain at the time of injection. Usually, the pain used to subside after about 1-5 min. Fever or myalgias was reported by three patients only and none of them required any analgesics or antipyretics for this purpose.
Of the total 34 patients available for analysis of study results, 19 (55.9%) showed a complete resolution of the treated as well as untreated warts [Table 1]. The usual scenario seen in these patients was a progressive reduction in the size of the treated wart that usually started after the first injection only with complete resolution by the third injection [Figures 1 and 2]. Simultaneously, with the changes in the surface area of the treated wart, the untreated warts also disappeared gradually without any inflammatory sequalae [Figure 3a and b].
Table 1.
Response to intralesional Candida injection immunotherapy

Figure 1a.
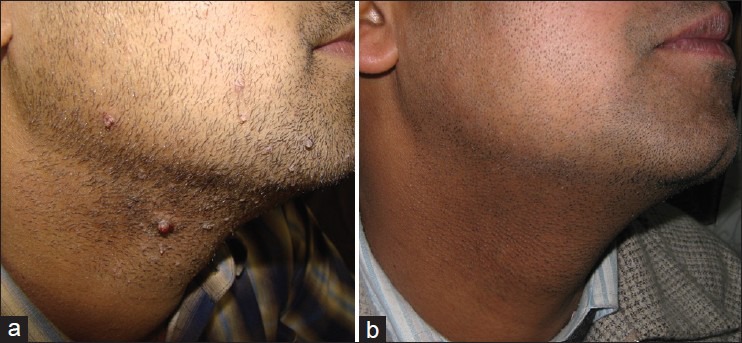
(a) Recurrent warts on face after multiple destructive procedures. (b) Same patient after Candida immunotherapy
Figure 2.
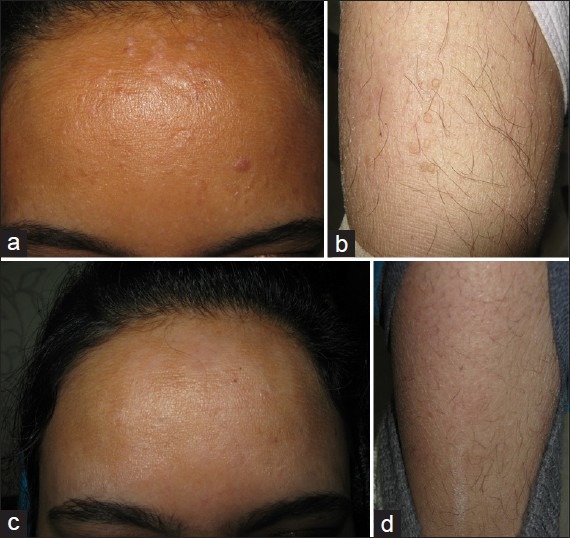
(a) Resistant warts on the face. (b) Concurrent warts on the leg. (c) Patient after Candida injections given into one facial wart. (d) Same patient showing resolution of distant warts on the leg
Figure 3.
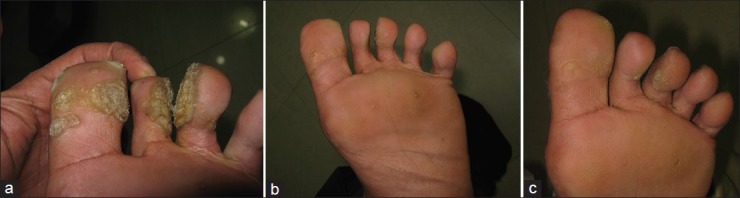
(a) Extensive veruccae on feet since > 8 years. (b) Partial resolution after two doses of Candida injection. (c) Almost complete resolution after three doses
In two more patients (6%), the treated wart was seen to respond partially to treatmentregimen, but there were no changes observed in the untreated warts. In these two patients, warts were seen on the face and hands and it was only the injected wart on the hand that showed a partial resolution after treatment. Both of these patients had multiple verruca plana on the face that failed to show any responseto the treatment regimen. Patients with facial verucca plana only were not treated with the test antigen injections. However, there were five patients who had verucca plana on the face in addition to the presence of common warts on either the hands or the feet. In these patients, only the mother wart on hands or feet was injected with the Candida test antigen. Three of these five patients showed a positive response to the treatment regimen with resolution of the plane warts on the face along with resolution of common warts on the extra-facial sites.
In the follow-up period of 6 months, none of the non-responders showed any further reduction in size or number of warts, and none of the patients in whom the warts had resolved with the treatment regimen showed any relapse anywhere on the body.
Technical difficulties
We encountered some technical difficulties while performing the intralesional injections and would like to point them here. The first and the most important is the possibility of the intralesional drug to ooze out of the wart while injecting. While injecting, the antigen solution tends to come out of the upper surface or the other side of the wart. This is especially common if one has to inject a wart of smaller size. To overcome this difficulty, it is better to inject the wart from the upper surface and not from the lateral side. Second, during the second and third injections, it becomes difficult to inject and accommodate the whole volume of the solution in some cases as the injected wart is already resolved to a great extent at that time.
Discussion
Treatment of warts is not always a simple affairas many patients fail to respond to all conventional modes of treatment. In addition, there are patients in whom warts do respond but there are multiple troublesome recurrences. The chances of a relapse are especially high when the warts are present on any part of the face. The lips and the area around the eyebrows in women and the shaving area in men are the most notorious for these troublesome recurrences to occur (personal observation). Repeating the same destructive procedure again and again in such patients is definitely not a feasible thing to do. Logically, the best possible treatment option in the above-mentioned cases is some form of immunotherapy that will boost the immune response to HPV infection in general.
Immunotherapy has been tried with oral immune modulators such as cimetidine[12,13] and levamisole[14] and also with topical immiquimod and intralesional interferons.[16,17] Immunotherapy with different skin test antigens like Candida, mumps, or trichophyton antigen is a relatively new treatment option for warts that has been studied over the last decade or so. The first antigen that was tried for immunotherapy of warts was that of Candida and the investigators reported success in majority of patients treated with this test antigen.[19,20] In a subsequent study, intralesional Candida injections were compared with cryotherapy in the treatment of warts.[21] The study reported complete resolution of the wart treated with Candida injections in 74% patients and complete resolution of even the untreated warts in 78% of these responders. Intralesional Candida immunotherapy has also been tried in children with recalcitrant warts with a response rate of 47% for the treated wart and 34% for all the body warts.[26] The effectiveness of Candida immunotherapy has even been described in genital warts.[27]
In addition to Candida, some other skin test antigens have been tried with success in treatment of warts. These include mumps antigen, trichophyton antigen, and even a combination of multiple antigens. Treatment with multiple antigens has, however, shown no advantages over single-antigen injections in terms of efficacy of treatment.[24]
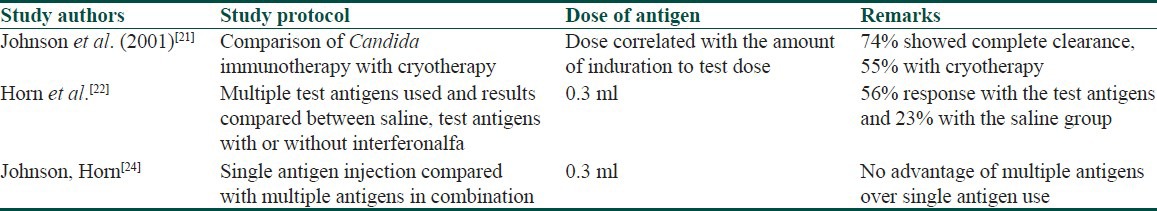
Different regimens have been described regarding the Candida antigen immunotherapy for warts [Table 2]. In one clinical study, the dose used has been correlated with the amount of induration observed after the test dose.[21] When the induration observed with the test dose antigen was more than 40 mm, the therapeutic dose administered was 0.1 ml on each occasion, whereas those showing an induration of 20-40 mm and 5-20 mm were given a dose of 0.2 ml and 0.3 ml, respectively.[21] We used a similar dose regimen in all patients regardless of the extent of induration achieved after the test dose injection. This dosage regimen seems to be equally effective as we also achieved a positive response in more than half of our patients. Similarly, some authors have used up to five injections at 3-week intervals, whereas we used only three injections at 3-week intervals for the study.[22] We found that the person who ultimately shows a resolution of the injected and/or distant warts shows some response after the first or at the most after the second injection only. Persons who fail to show any response after the first or even the second injection are less likely to respond after the third dose as well. Follow-up visit of these patients for the subsequent injections becomes a problem if they fail to see any visible results even after the third dose of Candida injection. The limiting factor in prescribing this treatment option is the absence of any reaction to the test dose of the antigen in a subset of patients. Such patients get automatically disqualified from receiving this treatment. However, patients showing a negative response to one antigen can be tested with other antigens solutions such as those of trichophytonor mumps.[22]
Table 2.
Comparison with select studies on test antigen immunotherapy
It is a well-known fact that trauma of any sort including the trauma associated with an intralesional injection can lead to a non-specific immune response against the wart antigens with subsequent resolution of the traumatized wart. However, going by the high proportion of patients showing a positive response to intralesional immunotherapy, there seems to be a definite role of the test antigens in resolution of warts. Even the eradication of untreated warts after injecting a single wart goes in favor of a systemic immune response against HPV after introduction of the test antigens.
Adverse effects that have been reported with Candida immunotherapy include febrile reactions, myalgias, erythema, and edema at the injection site and painful purple digit syndrome.[28] We did not encounter any of these adverse events except mild fever in few patients. However, majority of our patients did complain of pain at the time of injection, which was severe in some instances.
Conclusions
Candida immunotherapy seems to be a promising treatment option in patients in whom the warts are either resistant or not amenable to destructive modes of treatment. The treatment option is not expensiveand can be easily afforded by the patients. There is no risk of any scarring at the sites of intervention or at the site of warts. Furthermore, treatment of a single wart needs to be done even if there are hundreds of warts on the body. Risk of relapse also seems to be really low, and this is so important in patients suffering from troublesome recurrences of warts.
Limitations
Absence of a control group and small sample size of the study population were important limitations of the study. Therefore, we would definitely recommend more extensive, randomized controlled studies on this subject in larger population groups and with different dosage regimens to make it a routine treatment option to be offered to patients.
What is new?
Multiple intralesional injections with Candida test antigen is an effective treatment option in the treatment of recurrent or resistant warts.
A single uniform dose can be used for injection irrespective of the extent of induration with the test dose of the injection.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Sterling JC, Handfield-Jones S, Hudson PM. British Association of Dermatologists Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144:4–11. doi: 10.1046/j.1365-2133.2001.04066.x. [DOI] [PubMed] [Google Scholar]
- 2.alAboosi M. Treatment of plane warts by tretinoin-induced irritant reaction. Int J Dermatol. 1994;33:826–7. doi: 10.1111/j.1365-4362.1994.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs S, Harvey I, Sterling J, Stark R. Local treatments for cutaneous warts: Systematic review. BMJ. 2002;325:461. [PMC free article] [PubMed] [Google Scholar]
- 4.Savant SS, Gore D. Electrosurgery. In: Savant SS, Shah RA, Gore D, editors. Text Book and Atlas of Dermatosurgery and Cosmetology. Mumbai: ASCAD; 2005. pp. 305–14. [Google Scholar]
- 5.Connolly M, Bazmi K, O’Connell M, Lyons JF, Bourke JF. Cryotherapy of viral warts: A sustained 10-s freeze is more effective than the traditional method. Br J Dermatol. 2001;145:554–7. doi: 10.1046/j.1365-2133.2001.04449.x. [DOI] [PubMed] [Google Scholar]
- 6.Bourke JF, Berth-Jones J, Hutchinson PE. Cryotherapy of common viral warts at intervals of 1, 2 and 3 weeks. Br J Dermatol. 1995;132:433–6. doi: 10.1111/j.1365-2133.1995.tb08678.x. [DOI] [PubMed] [Google Scholar]
- 7.Krupashankar DS. Standard guidelines of care: CO 2 laser for removal of benign skin lesions and resurfacing. Indian J DermatolVenereol Leprol. 2008;74:S61–7. [PubMed] [Google Scholar]
- 8.Hruza GJ. Laser treatment of epidermal and dermal lesions. Dermatol Clin. 2002;20:147–64. doi: 10.1016/s0733-8635(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 9.Tan OT, Hurwitz RM, Stafford TJ. Pulsed dye laser treatment of recalcitrant verrucae: A preliminary report. Lasers Surg Med. 1993;13:127–37. doi: 10.1002/lsm.1900130120. [DOI] [PubMed] [Google Scholar]
- 10.Dhar SB, Rashid MM, Islam A, Bhuiyan M. Intralesionalbleomycin in the treatment of cutaneous warts: A randomized clinical trial comparing it with cryotherapy. Indian J Dermatol Venereol Leprol. 2009;75:262–7. doi: 10.4103/0378-6323.48428. [DOI] [PubMed] [Google Scholar]
- 11.Lewis TG, Nydorf ED. Intralesional bleomycin for warts: A review. J Drugs Dermatol. 2006;5:499–504. [PubMed] [Google Scholar]
- 12.Rogers CJ, Gibney MD, Siegfried EC, Harrison BR, Glaser DA. Cimetidine therapy for recalcitrant warts in adults: Is it any better than placebo? J Am Acad Dermatol. 1999;41:123–7. doi: 10.1016/s0190-9622(99)70421-4. [DOI] [PubMed] [Google Scholar]
- 13.Bauman C, Francis JS, Vanderhooft S, Sybert VP. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1996;35:271–2. doi: 10.1016/s0190-9622(96)90351-5. [DOI] [PubMed] [Google Scholar]
- 14.Parsad D, Saini R, Negi KS. Comparison of combination of cimetidine and levamisole with cimetidine alone in the treatment of recalcitrant warts. Australas J Dermatol. 1999;40:93–5. doi: 10.1046/j.1440-0960.1999.00328.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Morano T, del Boz J, González-Carrascosa M, Tortajada B, de Troya M. Topical cidofovir for viral warts in children. J Eur Acad Dermatol Venereol. 2011;25:1487–9. doi: 10.1111/j.1468-3083.2010.03961.x. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg NB, Lim JK, Paller AS, Mancini AJ. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42:803–8. doi: 10.1067/mjd.2000.103631. [DOI] [PubMed] [Google Scholar]
- 17.Muzio G, Massone C, Rebora A. Treatment of non-genital warts with topical imiquimod 5% cream. Eur J Dermatol. 2002;12:347–9. [PubMed] [Google Scholar]
- 18.Hengge UR, Esser S, Schultewolter T, Behrendt C, Meyer T, Stockfleth E, et al. Self-administered topical 5% imiquimod for the treatment of common warts and molluscum contagiosum. Br J Dermatol. 2000;143:1026–31. doi: 10.1046/j.1365-2133.2000.03777.x. [DOI] [PubMed] [Google Scholar]
- 19.Brunk D. Injection of Candida antigen works on warts. Skin Allergy News. 1999;30:5. [Google Scholar]
- 20.Phillips RC, Ruhl TS, Pfenninger JL, Garber MR. Treatment of warts with Candida antigen injection. Arch Dermatol. 2000;136:1274–5. doi: 10.1001/archderm.136.10.1274-a. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: A novel immunotherapy for warts. Arch Dermatol. 2001;137:451–5. [PubMed] [Google Scholar]
- 22.Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and trichophyton skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589–94. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- 23.Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268–71. doi: 10.1046/j.1525-1470.2003.20318.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens: A safe and effective therapy. J Drugs Dermatol. 2004;3:263–5. [PubMed] [Google Scholar]
- 25.Signore RJ. Candida albicans intralesional injection immunotherapy of warts. Cutis. 2002;70:185–92. [PubMed] [Google Scholar]
- 26.Maronn M, Salm C, Lyon V, Galbraith S. One-year experience with Candida antigen immunotherapy for warts and molluscum. Pediatr Dermatol. 2008;25:189–92. doi: 10.1111/j.1525-1470.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 27.King M, Johnson SM, Horn TD. Intralesional immunotherapy for genital warts. Arch Dermatol. 2005;141:1606–7. doi: 10.1001/archderm.141.12.1606. [DOI] [PubMed] [Google Scholar]
- 28.Perman M, Sterling JB, Gaspari A. The painful purple digit: An alarming complication of Candida albicans antigen treatment of recalcitrant warts. Dermatitis. 2005;16:38–40. [PubMed] [Google Scholar]


