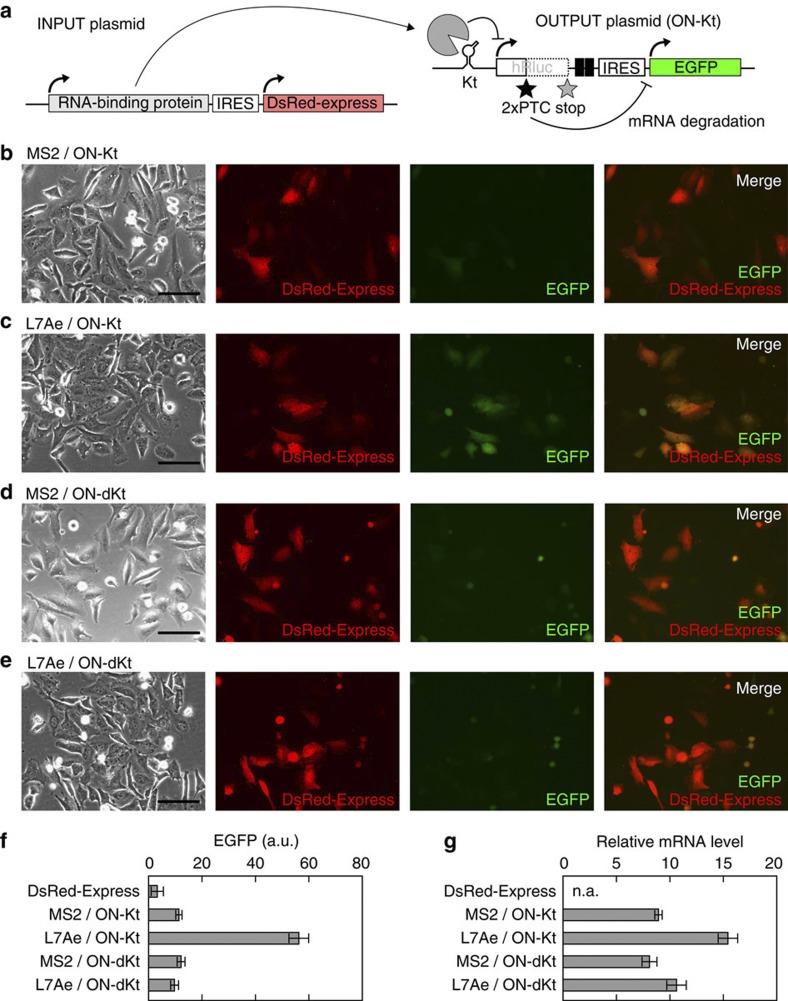
Figure 2. Behaviour of an inverted ON switch generated via insertion of the module into an OFF switch.
(a) Construction of the INPUT and OUTPUT plasmids transfected into HeLa cells in this study. The INPUT plasmids express either L7Ae or the MS2 coat protein (MS2) as a cognate or noncognate RNA-binding protein, respectively. DsRed-Express is also synthesized from the INPUT plasmid, driven by the IRES. An OUTPUT plasmid expresses ON-switch mRNAs containing K-turn (Kt) or defective K-turn (dKt) as either an active or defective sensory motif, respectively. EGFP driven by the IRES is synthesized from the OUTPUT plasmid. (b–e) Images of cells captured via fluorescence microscopy. DsRed-Express synthesized together with L7Ae (c,e) or MS2 (b,d) is shown in red. EGFP outputs from an ON switch with either an active (b,c; ON-Kt) or a defective (d,e; ON-dKt) sensor are shown in green. These two fluorescent signals are merged and shown in the right most pictures. Scale bars, 200 μm. (f) The mean intensity of EGFP fluorescence of transfected cells. HeLa cells shown in b–e were analysed using a flow cytometer, FACSAria. Cells expressing only DsRed-Express were used as the negative control (DsRed-Express). Bars and error bars represent the mean and s.d., respectively, of three triplicate experiments (n=9). (g) The levels of ON-switch mRNAs, as determined by quantitative RT–PCR. To eliminate the noise from untransfected cells, the results are normalized by employing the mRNA of neomycin-resistant gene transcribed from the OUTPUT plasmid in cells. Bars and error bars represent the mean and s.d., respectively, of three triplicate experiments (n=9). n.a., not analysed. All experiments in b–g were carried out 24 h after the transfection of 100 ng of the indicated OUTPUT plasmid and 20 ng of the INPUT plasmid together with 480 ng of the noncognate INPUT plasmid.