Abstract
Silymarin is an active principle from the seeds of the milk thistle plant and is widely used as a hepatoprotective gent due to its antioxidant-like activity. In the present study, we evaluated the potential efficacy of silymarin against oral cancer and investigated its possible mechanism of action. Cell viability assay and western blotting analyses were used to identify silymarin-induced apoptotic cell death in human pharynx squamous cell carcinoma (FaDu) cells. The short interfering RNA (siRNA) is used to confirm the role of phosphatase and tensin homolog (PTEN) in silymarin-induced apoptosis. Treatment of FaDu cells with silymarin resulted in a significant decrease in cell viability (up to 70%). Silymarin inhibited the phosphorylation of Akt (over 10-fold) with an increase in expression of PTEN (five to sixfold). Consequently, the level of Bcl-2 expression was decreased five to sixfold and caspase 3 activated to induce apoptosis. Treatment with siRNA specific to PTEN gene diminished the action of silymarin. The results suggest that silymarin inhibits the Akt signaling pathway by increasing PTEN expression in FaDu cells and directly affects Bcl-2 family members. Also, we demonstrated the inhibitory activity of silymarin for oral cancer is related to cell survival. These mechanisms may in part explain the actions of silymarin and provide a rationale for the development of silymarin as an anticancer agent.
Key Words: Akt signaling, Bad, Bcl-2, FaDu cells, PTEN, silymarin
Introduction
Silymarin is an active principle obtained from seeds of the milk thistle plant Silybum marianum (L.) Gaertn. (Asterceae). It contains ∼65–80% silymarin flavonolignans (silymarin complex) with small amounts of flavonoids and ∼20–35% fatty acids and other polyphenolic compounds.1 It is a mixture of flavonolignans, which are widely used as herbal medicine and/or dietary supplement in the treatment of alcoholic liver disease, acute and chronic viral hepatitis, or toxin-induced liver damage.2,3 Silymarin is known to be safe and well tolerated for protecting against liver injury.4–6 Silibinin is another active principle of milk thistle, the polyphenolic flavonoid that is a known antioxidant and/or free radical scavenger.7–9
Apoptosis is a physiological cell suicide program that can be evoked in response to stimuli such as ionizing radiation, toxins, and anticancer drugs. The induction of apoptosis is known to be an efficient and promising strategy to kill cancer cells.10 Many plant extracts and phytochemicals have been reported to induce apoptosis in cancer cell lines.11–13 The PI3K/Akt pathway is a pivotal signaling pathway, which controls cell growth, survival, proliferation, and tumor genesis.14,15 PI3K-activated (phosphorylated) Akt promotes cell survival by inhibiting apoptosis through its ability to phosphorylate/inactivate downstream targets of apoptotic machinery, such as anti-apoptotic Bcl-2 family member Bcl-2 and pro-apoptotic Bcl-2 family member BAD.16 The (PI3K)/Akt pathway is regulated by several critical upstream factors, for example, tumor suppressor phosphatase and tensin homolog (PTEN).17 The tumor suppressor gene PTEN is one of the most common targets of mutation in human cancers. Genetic mutation of PTEN resulted in increased Akt activity in many types of tumor.18 PTEN exerts a tight regulatory control over the PI3K pathway and downstream functions, including activation of protein kinase B/Akt, cell survival, cell proliferation, and cell migration.19,20 Inhibition of the PI3K/Akt pathway has been targeted as a strategy for drug development.21 For instance, some of the dietary phytochemicals have been shown to down regulate PI3K/Akt pathway and induced apoptosis.
Previous reports have shown that apoptosis can be induced by silibinin in hepatocellular carcinoma (HCC) by increasing production of caspase 3 or 9,22 or by inhibiting p-survivin.23 Also, it has been indicated that silibinin significantly increased PTEN expression and activity that was further associated with reduced p-Akt production in HCC xenograft tissue.24 However, the anti-cancer effect of silymarin related to PTEN expression is still obscure. The current study investigated the effect of silymarin on oral cancer and investigated the potential mechanism(s) relating to PTEN/PI3K/Akt pathway.
Head and neck squamous cell carcinomas of the oral cavity, pharynx, and larynx occur at a rate of ∼500,000 new cases per year worldwide25 and represent the sixth most common human neoplasm.26 One of the active components of silymarin, silibinin, has been reported to inhibit the activation of ERK1/2 in SCC-4 tongue cancer cells.27 Our previous report indicated that silymarin had the potential to suppress the survival, migration, and invasion of C-33A cancer cells in vitro.28 However, the effect of silymarin on oral cancer is still unknown. FaDu is derived from the human pharynx squamous cell carcinoma and the effect of silymarin on the squamous cell carcinoma from lower oral cavity is still unknown. Thus, we used it in this study.
Materials and Methods
Reagents
Silymarin, silibinin, and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Opti-MEM® I Reduced Serum Medium, Stealth™ Select RNAi (short interfering RNA [siRNA]-PTEN), scramble siRNA (siRNA-control), and Lipofectamine 2000™ were purchased from Invitrogen (Carlsbad, CA, USA). Antibodies against caspase 3, Bcl-2, p-Bad, and actin were purchased from Millipore (Bedford, MA, USA). Antibodies against Akt and phospho-Akt (Ser 473) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against PTEN were purchased from Abcam (Cambridge, United Kingdom).
Cell cultures
FaDu cells, a human pharynx squamous cell carcinoma cell line, were obtained from the Culture Collection and Research Center of the Food Industry Institute (Hsinchiu City, Taiwan), The FaDu cells were cultured in α-MEM (Hyclone, Logan, UT, USA) supplemented with 10% (v/v) FBS, 100 IU/mL penicillin (100 μg/mL) streptomycin, sodium pyruvate (0.11 mg/mL), and 1% (v/v) nonessential amino acids at 37°C in 5% CO2:95% air with high humidity. After ∼60% confluence, serum-free cell medium with varying final concentrations of silymarin (28.8, 38.4, 48, 57.6, and 67.2 μg/mL) were dispersed and cells were cultured for a further 24 h. The cells were harvested by treating with 0.25% trypsin with 0.2 g/L EDTA for further studies.
MTT assay for cytotoxicity
Cell viability and survival was determined by MTT (3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay.29 Briefly, 104 cells were plated in 96-well plates in triplicate and treated with different concentrations of the compounds during 6, 12, 24, and 48 h. After treatment, the medium was removed and replaced with 100 μL/well of fresh medium and 10 μL of MTT (final concentration 0.5 mg/mL) was added to each well. The plates were incubated at 37°C for 4 h, allowing viable cells to reduce the yellow tetrazolium salt into dark blue formazan crystals. The formazan crystals were dissolved using a solution of 0.01 M HCl/SDS 10%. Finally, the absorbance in each individual well was determined at 595 nm, absorbance of the cells counted by Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). The results of the assays were presented as mean±SEM. The data were collected from the time- and dose-responsed data of at least three independent experiments. Cells were treated with various concentrations of silymarin (0–140 μM) at the time point of 6, 12, 24, or 48 h, as described in the previous study.30
Western blot analysis
Protein was extracted from tissue homogenates and cell lysates using ice-cold radioimmunoprecipitation assay (RIPA) buffer supplemented with phosphatase and protease inhibitors (50 mM sodium vanadate, 0.5 mM phenylmethylsulphonyl fluoride, 2 mg/mL aprotinin, and 0.5 mg/mL leupeptin). Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total proteins (30 μg) were separated by SDS/polyacrylamide gel electrophoresis (10% acrylamide gel) using the Bio-Rad Mini-Protein II system. Protein was transferred to polyvinylidene difluoride membranes (PerkinElmer, Waltham, MA, USA) with a Bio-Rad Trans-Blot system. After transfer, the membranes were washed with phosphate-buffered saline (PBS) and blocked for 1 h at room temperature with 0.05 g/ml skimmed milk powder in PBS. Blots were incubated overnight at 4°C for the primary antibody reactions to bind the target protein such as PTEN. The blots were incubated with goat polyclonal antibody (1:1000) to bind the actin serving as the internal control. After the removal of primary antibody, the blots were extensively washed with PBS/Tween 20. The blots were then incubated for 2 h at room temperature with the appropriate peroxidase-conjugated secondary antibody diluted in PBS/Tween 20. The blots were developed by autoradiography using the enhanced chemiluminescence western blotting substrate (Amersham International, Buckinghamshire, United Kingdom). The immune blots were quantified with a laser densitometer (Avegene Life Science, Taipei, Taiwan).
Short interfering RNA
Duplexed RNA oligonucleotides for human PTEN (Stealth RNAi) were synthesized by Invitrogen. FaDu cells were transfected with 40 pmol of PTEN-specific siRNAs (siRNA-PTEN) or scrambled siRNA using serum-free Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols, and treated 48 h post-transfection. The sequences of the siRNA-PTEN were AUU GUC AUC UUC ACU UAG CCA UUG G (sense strand) and CCA AUG GCU AAG UGA AGA UGA CAA U (antisense strand) as described previously.31
Statistical analysis
Data are expressed as the mean±SEM in one group as indicated. Repeated measures analysis of variance (ANOVA) was used to analyze the changes in gene and protein expression or other parameters. A P value of .05 or less was considered significant.
Results
Cytotoxicity of silymarin against FaDu cells
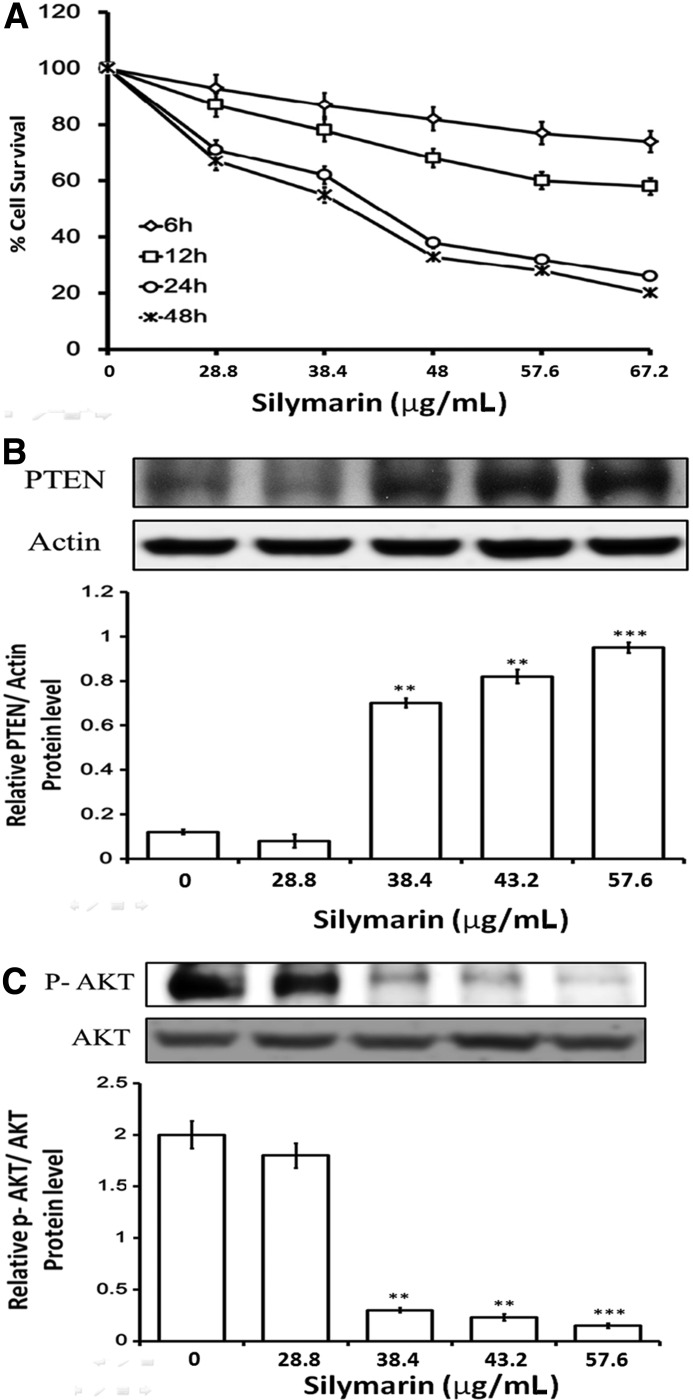
To screen the inhibitory effect of silymarin on FaDu cells, cytotoxicity assays were carried out as described above. Figure 1A shows the survival curves of FaDu cells treated with various concentrations of silymarin for different exposure times. The survival curves shifted to the left with longer drug exposures. The 50% inhibitory concentration values for silymarin treatment were estimated to be 28.8–67.2 μg/mL for 6, 12, 24, and 48 h, respectively. The control cells were treated with ethanol (0.1%), which did not affect cell proliferation, viability, or morphology (survival rate: 100% for 0 h, 99.34% for 6 h, 97.62% for 12 h, 95.40% for 24 h, and 93.41% for 24 h). These data indicate that silymarin exerts a significant cytotoxic effect on FaDu cells in a dose- and time-dependent manner. Also, the time point at 24 h and the dose of 57.6 μg/mL showed the plateau regarding the inhibitory effect of silymarin on FaDu cells. Thus, this time point and the dose were used in all experiments.
FIG. 1.
Silymarin inhibits Akt by enhancing PTEN expression and cytotoxicity in FaDu cells. Cell proliferation and viability were determined by an MTT assay. Reduced cell viability was observed with silymarin treatment (28.8–67.2 μg/mL) at 6, 12, 24, and 48 h (A). Proteins isolated from FaDu cells were probed with antibodies against PTEN (B), p-AKT, and AKT (C). The same membrane was re-probed with the antibody for β-actin to verify that equal amounts of protein were loaded. Data are expressed as the mean±SE (n=4 for each group). **P<.01 compared with control; ***P<.001 compared with control. PTEN, phosphatase and tensin homolog.
Silymarin inhibits Akt by enhancing PTEN expression
The effects of silymarin on expression of PTEN were examined by western blot analysis. As shown in Figure 1B and C, the expression level of phosphorylated AKT was decreased with an increase of PTEN in FaDu cells treated with silymarin for 24 h in a concentration-dependent manner.
Silymarin induces caspase-dependent cell apoptosis
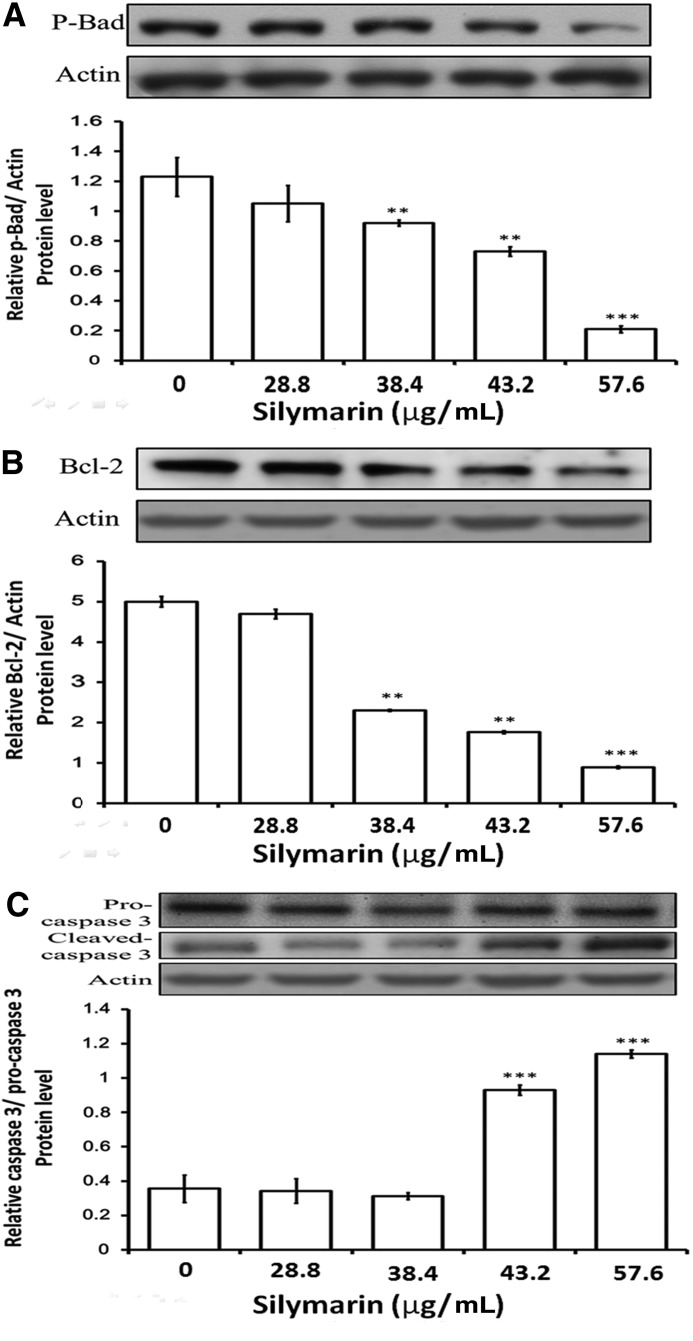
Apoptosis is a type of programmed cell death that is caspase dependent.32 To determine whether silymarin-induced apoptosis was caspase 3 dependent, the levels of pro-caspase 3, cleaved-caspase 3, and Bcl-2 were visualized by western blot of protein extracts from cells treated with silymarin. A concentration-dependent decrease in the levels of Bcl-2 and p-Bad was observed in FaDu cells when treated with silymarin (Fig. 2). The increase of cleaved-caspase 3 was also observed. These results indicate that silymarin-induced cell death mediated caspase-dependent apoptosis.
FIG. 2.
Silymarin treatment resulted in altered levels of apoptosis-associated proteins. (A) FaDu cells were treated with silymarin, and then protein samples were probed with antibodies against p-Bad (A), Bcl-2 (B), por-caspase 3, and caspase 3 (C). The same membrane was re-probed with the antibody for β-actin to verify that equal amounts of protein were loaded. Data are expressed as the mean±SE (n=4 for each group). **P<.01 compared with control; ***P<.001 compared with control.
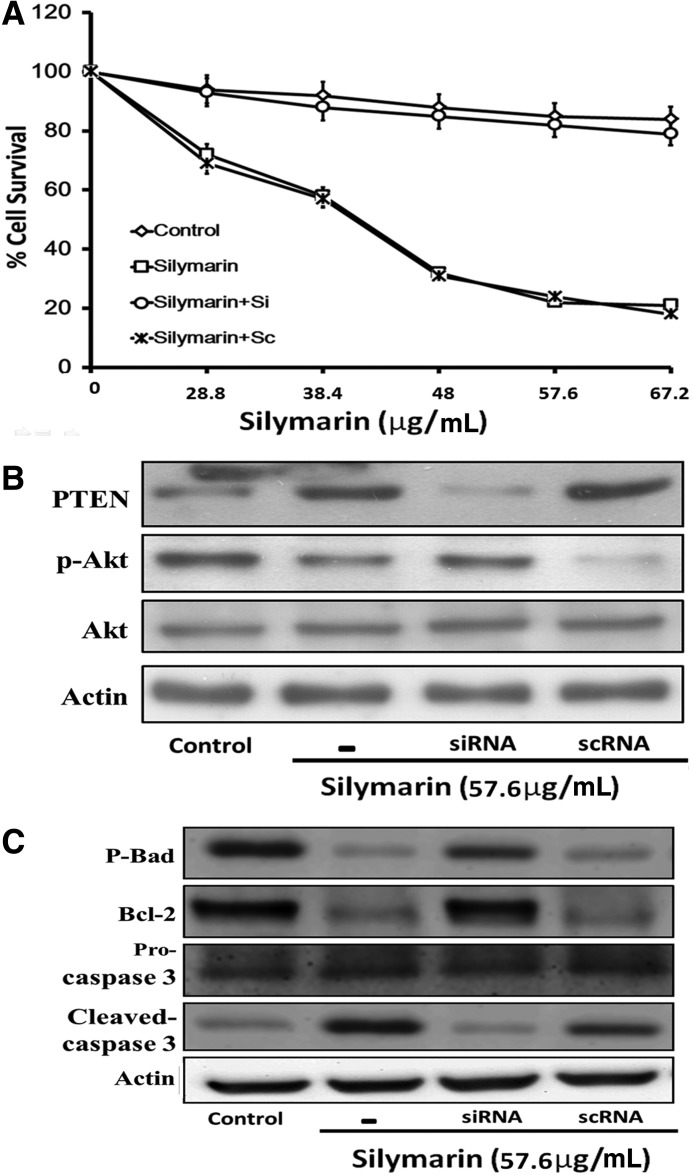
Silencing of the PTEN gene diminished the apoptotic effect of silymarin in FaDu cells
In the current study, we observed that the protein level of PTEN increased in FaDu cells by silymarin and the level of p-Akt also decreased. Consequently, the cells underwent apoptotic cell death. To identify the key role of PTEN in silymarin-induced apoptosis, we applied siRNA of PTEN in FaDu cells. As shown in Figure 3, the cytotoxic effects of silymarin (28.8–67.2 μg/mL for 24 h) on FaDu cells were attenuated in cells receiving siRNA of PTEN (P<.001 compared with control, for silymarin and silymarin with scRNA group). According to Figure 1, the time point at 24 h and the dose of 57.6 μg/mL reached a plateau for the inhibitory effect of silymarin on FaDu cells. We used this time point and dose in this part of study. The levels of p-Akt (P<.001 compared with control, for silymarin and silymarin with scRNA group), p-Bad (P<.001 compared with control, for silymarin and silymarin with scRNA group), caspase 3 (P<.001 compared with control, for silymarin and silymarin with scRNA group), and Bcl-2 (P<.001 compared with control, for silymarin and silymarin with scRNA group) were also reversed by the treatment of siRNA (Fig. 3B, C).
FIG. 3.
PTEN siRNA attenuated cytotoxicity and PTEN expression in FaDu cells treated with silymarin. Cell proliferation and viability were determined by an MTT assay (A). After treated with silymarin 57.6 μg/mL for 24 h, proteins isolated from FaDu cells were probed with antibodies against PTEN, p-AKT, and AKT (B). Proteins isolated from FaDu cells were probed with antibodies p-Bad, Bcl-2, and caspase 3 and pro-caspase 3 (C). siRNA, short interfering RNA.
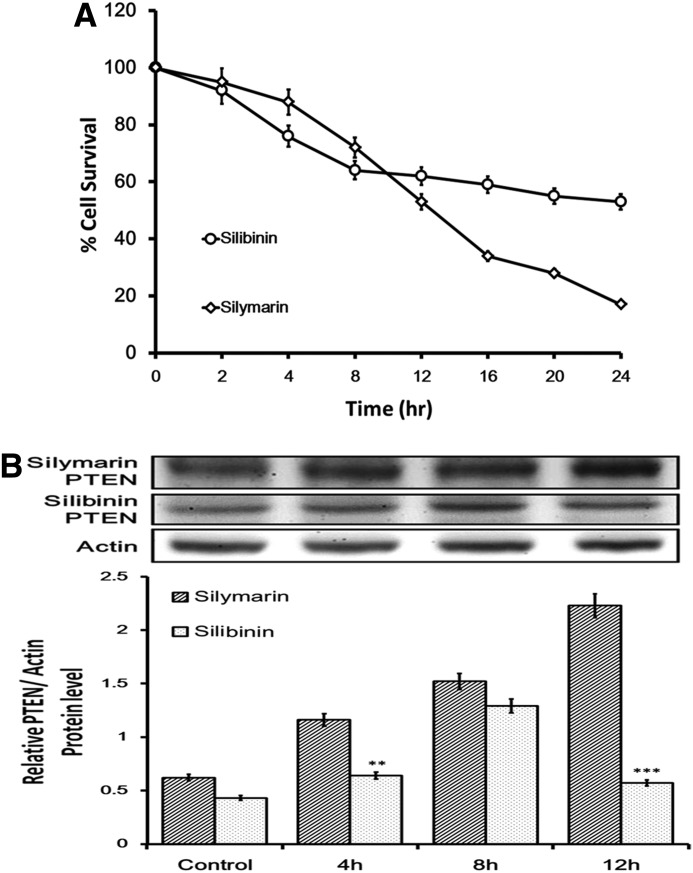
Silymarin is a more potent inducer of apoptotic cell death than silibinin
Cytotoxic effects of silymarin and silibinin on FaDu cells were examined. To identify the inhibitory effect of silymarin and silibinin on FaDu cells, cytotoxicity assays were carried out as described above. Also, the potency between silymarin and silibinin was compared from the used dose. Figure 4A shows the survival curves of FaDu cells treated with 57.6 μg/mL of silymarin, which is more marked than 96.4 μg/mL of silibinin showing as effective dose from previous reports22,23,33 at different exposure times (0–24 h). These data indicated that silymarin exerts much stronger cytotoxic effect than silibinin on FaDu cells. We further tested the expression level of PTEN induced by silymarin or silibinin. Figure 4B shows that both silymarin and silibinin could increase PTEN in FaDu cells at the time point of 4 h. However, increased expression of PTEN by silibinin is soon diminished at the time point of 12 h, but the effect of silymarin is continuously expressed until the end of experiment.
FIG. 4.
Comparison of cytotoxic effects of silymarin and silibinin on FaDu cells. Cell proliferation and viability were determined by an MTT assay. Reduced cell viability was observed with silymarin (57.6 μg/mL) or silibinin (96.4 μg/mL) treatment at 0–24 h (A). Proteins isolated from FaDu cells were probed with antibodies against PTEN (B). The same membrane was re-probed with the antibody for β-actin to verify that equal amounts of protein were loaded. **P<.01 compared with control; ***P<.001 compared with control.
Discussion
In the present study, we investigated the direct cytotoxicity of silymarin in human pharynx squamous cell carcinoma cell line (FaDu cells). Silymarin increased PTEN expression to result in a significant inhibition of phosphorylated Akt (at Ser473) in these tumor cells. Moreover, the loss of Bad phosphorylation and higher cell apoptosis were observed. The actions of silymarin including a decrease in anti-apoptotic proteins and PI3K/Akt pathway inactivation were both reversed by silencing of PTEN gene through siRNA. It has also been suggested that silibinin, another component of milk thistle extract, significantly increases PTEN expression resulting in a decrease of p-Akt production in hepatic tumor.24 This action of silymarin is consistent with that observed in cervical cancer cell.28 However, in the present study, silymarin showed a higher potency than silibinin to induce apoptotic cell death in oral cancer cells. Also, the current study confirmed that silymarin is effective against more than one cancer cell type. Thus, silymarin is suitable for the development of anticancer drugs for oral cancers, which need effective agents now.34
PTEN mutation in some cancer cells was found with distant metastases or tumor invasions, suggesting that PTEN mutation is a late-stage event that may contribute to an invasive and metastatic tumor phenotype.35 A prominent phosphorylation of Bad was observed also, thus suggesting that pro-apoptotic Bad protein was inactivated. It is conceivable that phosphorylated Bad may play a role in the survival of cancer cells and that dephosphorylation by Akt inhibitor may induce cancer cells to undergo apoptosis. Bad phosphorylation may be closely related to aberrant survival, namely, carcinogenesis. In various types of human malignant tumors, an elevated Akt activation has been demonstrated and the mechanisms for Akt activation have been shown to depend on the types of tumors. For example, ovarian carcinomas and endometrial carcinoma have shown to increase Akt activation due to a high frequency of PTEN inactivation.18,36 In 2005, an article offered new insights into the regulation of the PTEN/AKT pathway and the mechanisms of resistance to tumor genesis. This article also suggested that inhibitors of the AKT pathway should be tested and potentially be developed as new therapeutic agents.37 In the present study, we found that silymarin can inhibit AKT pathway by increasing PTEN. Finally, if the anti-apoptotic protein Bad is inactivated it may directly trigger caspase-dependant cell death.
Silymarin at concentrations of 24–48 μg/mL induced apoptotic death by inhibiting Akt activity38 or the p53-dependent pathway,39 decreasing cell viability by 40–20%.38 In contrast to silymarin, silibinin caused cell cycle arrest in the G1 phase in human colon carcinoma HT-29 cells and inhibited cell proliferation rate at concentrations of 75–100 μg/mL (about 150–200 μM)33 that is higher than the effective dose of silymarin (<48 μg/mL). Also, suppression of growth in human colorectal carcinoma (SW480) by silibinin has been observed at concentrations of 200 μM.40 It is clear that silymarin has higher potency than silibinin to induce apoptotic cell death. Silibinin is a compound purified from silymarin. However, direct increase of PTEN gene by silymarin is still not observed in silibinin-treated cells. Our study shows that increased expression of PTEN by silibinin is diminished within 4 h, but the effect of silymarin continued until the end of experiment. The result of cytotoxicity assays also reflects that silymarin has higher potency than silibinin to induce apoptotic cell death. This point was not mentioned before. However, the pharmacokinetic factors may be important and more in vivo studies are warranted to understand the stability and bioavailability of silymarin for the clinical applications.
Silybin A, silybin B, isosilybin A, isosilybin B, silydianin, silychristin, and isosilychristin were other components isolated from silymarin and they were used to screen the effect on prostate cancer cells.7 Then, isosilybin B (one of the active components) was found to cause cell cycle arrest.41 This may explain why silymarin is more effective for inducing cancer cell apoptosis.
In conclusion, the present study shows, for the first time, that silymarin has a higher potency than silibinin to induce apoptotic cell death in oral cancer. Also, silymarin inhibits the Akt signaling pathway by increasing PTEN expression in FaDu cells and directly affects Bcl-2 family members without other factors. These results provide a rationale for the development of silymarin as a therapeutic agent for oral cancer.
Acknowledgments
We appreciate Miss Shan-Yuan Liang and Pei-Ling Chou for research assistance. The present study is supported in part by a grant from Chi-Mei Medical Center, Tainan City, Taiwan (CMFHT9801).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kroll DJ. Shaw HS. Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 2.Feher J. Lengyel G. [Silymarin in the treatment of chronic liver diseases: past and future] Orv Hetil. 2008;149:2413–2418. doi: 10.1556/OH.2008.28519. [DOI] [PubMed] [Google Scholar]
- 3.Saller R. Meier R. Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 4.Flora K. Hahn M. Rosen H. Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramasamy K. Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyrou M. Bourgoin L. Foti M. PTEN in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and cancer. Dig Dis. 2010;28:236–246. doi: 10.1159/000282095. [DOI] [PubMed] [Google Scholar]
- 7.Singh RP. Agarwal R. A cancer chemopreventive agent silibinin, targets mitogenic and survival signaling in prostate cancer. Mutat Res. 2004;555:21–32. doi: 10.1016/j.mrfmmm.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs BP. Dennehy C. Ramirez G. Sapp J. Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med. 2002;113:506–515. doi: 10.1016/s0002-9343(02)01244-5. [DOI] [PubMed] [Google Scholar]
- 9.Lieber CS. Leo MA. Cao Q. Ren C. DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336–339. doi: 10.1097/00004836-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Bold RJ. Termuhlen PM. McConkey DJ. Apoptosis, cancer and cancer therapy. Surg Oncol. 1997;6:133–142. doi: 10.1016/s0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- 11.Yoon Y. Kim YO. Jeon WK. Park HJ. Sung HJ. Tanshinone IIA isolated from Salvia miltiorrhiza BUNGE induced apoptosis in HL60 human premyelocytic leukemia cell line. J Ethnopharmacol. 1999;68:121–127. doi: 10.1016/s0378-8741(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 12.Seo WG. Pae HO. Oh GS, et al. Ethyl acetate extract of the stem bark of Cudrania tricuspidata induces apoptosis in human leukemia HL-60 cells. Am J Chin Med. 2001;29:313–320. doi: 10.1142/S0192415X01000332. [DOI] [PubMed] [Google Scholar]
- 13.Lien HM. Lin HW. Wang YJ, et al. Inhibition of anchorage-independent proliferation, G0/G1 cell-cycle regulation in human colorectal carcinoma cells by 4,7-dimethoxy-5-methyl-l,3-benzodioxole isolated from the fruiting body of Antrodia camphorate. Evid Based Complement Alternat Med. 2011 doi: 10.1093/ecam/nep020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke TF. Hornik CP. Segev L. Shostak GA. Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 15.Franke TF. Yang SI. Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 16.Franke TF. Cantley LC. Apoptosis. A Bad kinase makes good. Nature. 1997;390:116–117. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- 17.Cantley LC. Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurose K. Zhou XP. Araki T. Cannistra SA. Maher ER. Eng C. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol. 2001;158:2097–2106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung JH. Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 20.Parsons R. Human cancer, PTEN and the PI-3 kinase pathway. Semin Cell Dev Biol. 2004;15:171–176. doi: 10.1016/j.semcdb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Workman P. Clarke PA. Raynaud FI. van Montfort RL. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res. 2010;70:2146–2157. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varghese L. Agarwal C. Tyagi A. Singh RP. Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res. 2005;11:8441–8448. doi: 10.1158/1078-0432.CCR-05-1646. [DOI] [PubMed] [Google Scholar]
- 23.Lah JJ. Cui W. Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol. 2007;13:5299–5305. doi: 10.3748/wjg.v13.i40.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui W. Gu F. Hu KQ. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J Gastroenterol. 2009;15:1943–1950. doi: 10.3748/wjg.15.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vokes EE. Weichselbaum RR. Lippman SM. Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 26.Landis SH. Murray T. Bolden S. Wingo PA. Cancer statistics. CA Cancer J Clin. 1999;1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Chen PN. Hsieh YS. Chiang CL. Chiou HL. Yang SF. Chu SC. Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J Dent Res. 2006;85:220–225. doi: 10.1177/154405910608500303. [DOI] [PubMed] [Google Scholar]
- 28.Yu HC. Chen LJ. Cheng KC. Li YX. Yeh CH. Cheng JT. Silymarin inhibits cervical cancer cell through an increase of phosphatase and tensin homolog. Phytother Res. 2012;26:709–715. doi: 10.1002/ptr.3618. [DOI] [PubMed] [Google Scholar]
- 29.Scudiero DA. Shoemaker RH. Paull KD, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 30.Matsuda T. Ferreri K. Todorov I, et al. Silymarin protects pancreatic beta-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology. 2005;146:175–185. doi: 10.1210/en.2004-0850. [DOI] [PubMed] [Google Scholar]
- 31.Poliseno L. Salmena L. Zhang J. Carver B. Haveman WJ. Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvesen GS. Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal C. Singh RP. Dhanalakshmi S, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 34.Nukatsuka M. Saito H. Sakamoto K, et al. Efficacy of combination chemotherapy using oral fluoropyrimidine S-1 with oxaliplatin (SOX) against colorectal cancer in vivo. Anticancer Res. 2012;32:2807–2812. [PubMed] [Google Scholar]
- 35.Lu Y. Lin YZ. LaPushin R, et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 1999;18:7034–7045. doi: 10.1038/sj.onc.1203183. [DOI] [PubMed] [Google Scholar]
- 36.Kanamori Y. Kigawa J. Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001;7:892–895. [PubMed] [Google Scholar]
- 37.Palomero T. Dominguez M. Ferrando AA. The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle. 2008;7:965–970. doi: 10.4161/cc.7.8.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong X. Zhu Y. Lu Q. Zhang J. Ge Z. Zheng S. Silymarin causes caspases activation and apoptosis in K562 leukemia cells through inactivation of Akt pathway. Toxicology. 2006;227:211–216. doi: 10.1016/j.tox.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 39.Katiyar SK. Roy AM. Baliga MS. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Ther. 2005;4:207–216. [PubMed] [Google Scholar]
- 40.Kaur M. Velmurugan B. Tyagi A. Agarwal C. Singh RP. Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia. 2010;12:415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deep G. Oberlies NH. Kroll DJ. Agarwal R. Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. Int J Cancer. 2008;123:41–50. doi: 10.1002/ijc.23485. [DOI] [PubMed] [Google Scholar]