Abstract
Objective
Depression and obesity are associated, but the impact of obesity on depression treatment outcome, or, conversely, the impact of treatment on body mass index (BMI) in depressed adolescents has not been reported. In this article, we examine the bidirectional relationships between BMI and treatment response in adolescents with treatment-resistant depression.
Method
Participants in the Treatment of Selective Serotonin Reuptake Inhibitor (SSRI) Resistant Depression in Adolescents (TORDIA) study had height and weight assessed at baseline, weekly for the first 6 weeks, biweekly for the next 6 weeks, and monthly from weeks 12 through 24. The impact of baseline BMI as a predictor and moderator of treatment response was assessed. In addition, participants' changes in BMI were assessed as a function of specific treatment assignment and treatment response.
Results
Participants assigned to SSRIs had a greater increase in BMI-for-age-sex z-score and weight than did those assigned to venlafaxine. Post-hoc, those treated with paroxetine or citalopram had the biggest increases in BMI, relative to fluoxetine or venlafaxine. Overweight or obesity was neither a predictor nor a moderator of treatment outcome, nor of subsequent BMI change.
Conclusions
Overweight status does not appear to affect treatment response in adolescents with resistant depression. The successful treatment of depression does not appear to favorably affect weight or BMI. Fluoxetine and venlafaxine are less likely to cause an increase in BMI than paroxetine or citalopram.
Introduction
Obesity and depression are two of the leading public health concerns (Simon et al. 2006). In the United States, 12–15% of the adolescent population is categorized as obese (Richardson et al. 2006; Boutelle et al. 2010;), and up to 1 in 5 adolescents will have experienced at least one major depressive episode by age 18 (Lewinsohn et al. 1998).
Depression and obesity frequently co-occur, possibly because of shared risk factors, such as early childhood adversity (Felitti et al. 1998; Stunkard et al. 2003; McIntyre et al. 2012). Depression may also lead to obesity, with the effect possibly more pronounced in girls (Richardson et al. 2003). Body mass index (BMI) shows accelerated growth after a depressive episode during adolescence (Pine et al. 2001). This association might be explained by decreased physical activity, increased appetite associated with depression, or weight gain associated with side effects of medications used to treat depression (Stunkard et al. 2003; Patten et al. 2009; Hasnain et al. 2012). Conversely, obesity may increase the risk for depression, possibly mediated by body shape dissatisfaction, peer or parental teasing, and decreased self-esteem, or because of incipient metabolic syndrome (Young-Hyman et al. 2006; Libbey et al. 2008; Wang et al. 2009; Bang et al. 2012; Hamer et al. 2012; Sanchez-Villegas et al. 2013). However, some longitudinal studies do not find that obesity predicts future depression (Gariepy et al. 2010).
Obesity and overweight may also affect treatment response. One study examined adults with depression participating in phase II–IV trials with selective serotonin reuptake inhibitors (SSRIs), and found that obesity was associated with a less vigorous treatment response, with the effect more pronounced in males (Khan et al. 2007). In the Munich Antidepressant Response Study (MARS), higher BMI was associated with a slower clinical response as well as a lower likelihood of normalization in tests of attention and of cortisol dynamics (Kloiber et al. 2007). In the Genome Based Therapeutic Drugs for Depression (GENDEP) study, obesity was associated with a poorer response to nortriptyline in men and women, and a poorer response to escitalopram in women (Uher et al. 2009). The mechanisms to explain the association between treatment resistance and higher BMI are not established, but might be explained by shared risk factors such as early adversity, which have shown to be associated with treatment resistance in depression (Nanni et al. 2012). To the extent that treatment response is sensitive to the blood concentration of antidepressants, obese patients may be more likely to have an inadequate drug concentration, which could in turn predict poor response (Hiemke 2008; Sakolsky et al. 2011; Ostad Haji et al. 2012). Another possible explanation for treatment resistance in obese patients is that the comorbid medical conditions, such as sleep apnea, asthma, and metabolic syndrome may lead to more severe depression and impairment, and render patients less likely to respond to treatment (Chapman et al. 2005; Moussavi et al. 2007).
Although there have been some investigations of the impact of treatment of depression on BMI, and, conversely, the impact of BMI on treatment response, to our knowledge, these relationships have never been studied in adolescents with depression. In one placebo-controlled trial, depressed adolescents treated with fluoxetine gained less weight and height than those randomized to placebo, but BMI was not reported (Nilsson et al. 2004). Given the paucity of data about the bidirectional relationship between adolescent depression treatment response and obesity, we endeavored to test the following hypotheses in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study: 1) A history of abuse, greater duration of depression, and symptoms of increased appetite, poor sleep, and decreased activity would be related to higher BMI at baseline, and a greater increase in BMI over time; 2) higher BMI would predict a poorer response overall and to citalopram and fluoxetine, two agents for which there was a relationship between drug concentration and outcome (Sakolsky et al. 2011); 3) those who remitted would have a more favorable trajectory of BMI and weight; and 4) participants treated with SSRIs, specifically paroxetine, would show more weight gain than those treated with venlafaxine.
Method
Design, methods, and results of treatment efficacy, as well as predictors and moderators of response, in the Treatment of Resistant Depression in Adolescence (TORDIA) study at 12 and 24 weeks, have been reported in detail in previous publications (Brent et al. 2008; Asarnow et al. 2009; Emslie et al. 2010). In summary, the TORDIA study was a six site randomized, controlled trial using a 2×2 balanced factorial design (Brent et al. 2008). Participants who did not respond to a previous adequate treatment trial for major depressive disorder (MDD) or dysthymic disorder were randomly assigned to switch either to an alternative SSRI (paroxetine, citalopram, or fluoxetine) or to venlafaxine, a selective serotonin-norepinephrine reuptake inhibitor (SNRI), with or without the addition of cognitive behavior therapy (CBT). The study was approved by each site's local institutional review board. All participants and their parents gave written informed assent/consent in accordance with local institutional review board regulations.
Participants
Participants included 334 adolescents (ages 12–18 years) with American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) MDD or dysthymia that persisted despite an adequate, 8 week treatment with an SSRI, the last four of which were at a dosage equivalent of at least 40 mg of fluoxetine (American Psychiatric Association 1994; Brent et al. 2008). Exclusion criteria included the following: Participants who had two or more adequate trials of an SSRI or a history of nonresponse to venlafaxine (≥4 weeks at a dosage of ≥150 mg) or CBT (≥7 sessions). Participants taking antipsychotics, mood stabilizers, or non-SSRI antidepressants were also excluded, with the exception of individuals receiving stable doses (≥12 weeks' duration) of stimulants, use of hypnotics (trazodone, zolpidem, zaleplon), or anti-anxiety agents (clonazepam, lorazepam). Diagnoses of bipolar spectrum disorder, psychosis, pervasive developmental disorder or autism, eating disorders, substance abuse or dependence, or hypertension (diastolic blood pressure ≥90) were also exclusionary (Brent et al. 2008; Emslie et al. 2010).
Height and weight were collected weekly for the first 5 weeks, biweekly from weeks 6–12, and monthly from weeks 12–24, for a total of 12 measurements, using the same type of scale and stadiometer at each visit.
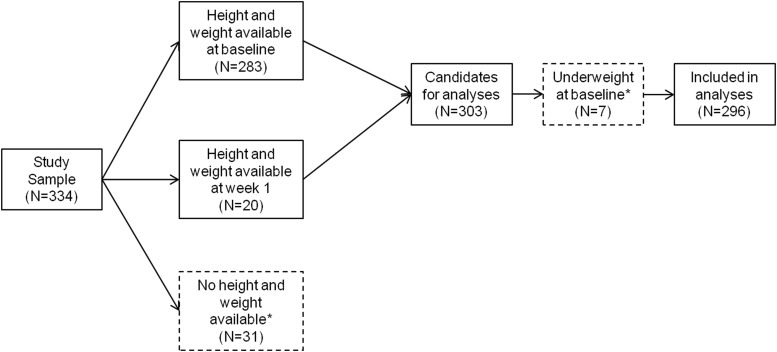
BMI, BMI-for-age-sex percentiles, and BMI-for-age-sex z-scores (BMI-z) were calculated using a SAS program provided by the Centers for Disease Control and Prevention (CDC) (Centers for Disease Control and Prevention 2011). Percentiles are based on the CDC Growth Reference Chart (Year 2000). Participants (see Fig. 1) were classified as underweight (BMI<5th percentile), normal (5th≤BMI≤85th percentile), overweight (85th<BMI≤95th percentile) or obese (BMI>95th percentile), based on accepted definitions (Krebs et al. 2007). Two hundred and eighty-three participants had height and weight information at baseline and were included in the BMI calculation; an additional 20 were included whose data were missing at baseline but available at week 1. Thirty-one participants were completely missing this information, and were therefore excluded. Only seven participants were classified as underweight at baseline; given the small size of this group, they were excluded from the analyses. Their demographic and clinical characteristics were similar to those of the 296 participants included (p's>0.06) except for lower parentally reported family conflict (9.5 [SD=6.0] vs. 13.0 [SD=2.4], t=−3.60, df=8.07, p=0.007); their response rates did not differ (p>0.99), however they were less likely to be remitters (0.0% vs. 39.2%, Fisher's exact test, p=0.046). Compared with the 38 excluded, the 296 participants included in this report had earlier age of onset of depressive episode (13.8 [SD=2.2] vs. 14.8 [SD=1.9], t=2.72, df=324, p=0.007), less suicidal ideation (40.7 [SD=21.9] vs. 48.8 [SD=24.3], t=2.04, df=326, p=0.04), and less hopelessness (10.3 [SD=5.5] vs. 12.2 [SD=6.2], t=2.01, df=325, p=0.046). There were no differences in the rates of response (p=0.47) or remission (p=0.78). They were of mid-adolescent age (mean age=15.8 years [SD=1.6]), with a majority of them being female (n=207 [69.9%]) and Caucasian (n=248 [83.8%]). One hundred and fifty were randomized to an SSRI (paroxetine, n=47; citalopram, n=31; fluoxetine, n=72), and 146 to venlafaxine.
FIG. 1.
Study flow of participants. Dashed boxes represent participants excluded from the analyses.
The mean number of available height and weight measurements was 7.0 (SD=3.9); the median was 8 (range 1–12). Once a participant was determined to be a nonresponder, weight and height were no longer recorded, which consisted of 51.7%, (n=153) of the sample in this report. Characteristics of those who had fewer than the median number of BMI assessments were compared with those with at least the median: there were no significant differences between the groups on BMI (25.4 [SD=6.7] vs. 26.0 [SD=6.6], t=−0.75, df=294, p=0.46), but those with fewer than the median number of height/weight assessments were older (16.1 years [SD=1.5] vs. 15.6 years [SD=1.6], t=2.85, df=293.4, p=0.005) and had fewer MDD episodes (1.2 [SD=0.5] vs. 1.4 [SD=0.8], t=−2.95, df=258.64, p=0.003); they were also less likely to be responders (39.9% vs. 56.2%, χ21=7.91, p=0.005). In addition, they had characteristics related to nonresponse, that is, greater impairment, (49.6 [SD=7.5] vs. 51.7 [SD=7.9], t=−2.34, df=291.8, p=0.02), higher family conflict (9.8 [SD=6.6] vs. 8.1 [SD=5.7], t=2.34, df=288, p=0.02), and higher rates of previous suicide attempts (28.7% vs. 18.4%, χ21=4.32, p=0.04) and non-suicidal self-injury (NSSI) (45.0% vs. 29.1%, χ21=7.86, p=0.04) (Asarnow et al. 2009). Change in BMI-z was not related to number of assessments (number of assessments by time, z=−0.21, p=0.84), meaning that the estimates of the change in BMI-z scores was not affected by the number of assessments.
Response/remission
DSM-IV diagnoses were assessed at baseline, 12, and 24 weeks using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version criteria (K-SADs-PL) (Kaufman et al. 1997; American Psychiatric Association 1994). The interviewer-rated Children's Depression Rating Scale-Revised (CDRS-R) was used to assess severity of depression at baseline and weeks 6, 12, and 24 (Poznanski et al. 1985). Clinical severity and improvement were rated using the Clinical Global Impressions Severity (CGI-S) and Improvement (CGI-I) subscales, respectively (Guy 1976). These diagnostic and global impression ratings assessments were conducted by an independent evaluator who was blind to treatment assignments. Response was defined as a CGI-I score of ≤2 and an improvement in the CDRS-R score ≥50%. Remission was defined as ≥3 consecutive weeks without clinically significant depressive symptoms with a score of 1 on the Adolescent Longitudinal Interval Follow-Up Evaluation (A-LIFE) (Keller et al. 1987). Physical and sexual abuse were recorded from the trauma screen for posttraumatic stress disorder on the K-SADS-PL.
Self-reported measures
The Beck Depression Inventory (BDI) (Beck et al. 1988), Beck Hopelessness Scale (BHS) (Beck et al. 1974), Suicidal Ideation Questionnaire–Junior High School version (SIQ-Jr) (Reynolds and Mazza 1999), and the Screen for Anxiety Related Disorders (SCARED) (Birmaher et al. 1997) were used at baseline, 6, 12, and 24 weeks to assess self-reported depression, hopelessness, suicidality, and anxiety symptoms respectively. Drug/alcohol use was assessed using the Drug Use Screening Inventory (DUSI) (Kirisci et al. 1995). Family conflict was assessed by the Conflict Behavior Questionnaire, adolescent and parent versions (CBQ-A and CBQ-P; Robin and Weiss 1980).
Medication blood levels
Plasma concentrations of study medication and metabolites were measured at 6 weeks in 244 participants, 207 of whom had BMI data. The geometric mean (GM) was used to evaluate the relationship between drug concentration and outcome as reported previously in Sakolsky et al. (2011).
Statistical analysis
Participants were initially categorized into three groups: normal, overweight, and obese, and differences in demographic, clinical, and treatment characteristics were compared using standard parametric or nonparametric univariate statistics. Multinomial logistic regression was then used to examine the most parsimonious set of variables that differentiated among the three BMI classes. The rates of response and remission were compared among the three groups using χ2 statistics, and then by logistic regression adjusting for covariates that differentiated among the three groups. To test for possible differential response of treatment as a function of BMI, a logistic regression was conducted testing for the effects of treatment, BMI category, and their interaction on treatment response. Conversely, to examine the impact of treatment on BMI, we examined the change in BMI-z as a function of response and remission, as well as the differential impact of treatment on change in BMI using random mixed effects regression. We also examined the impact of BMI on treatment outcome using scalar outcomes, with the CDRS-R and BDI, using general linear mixed models. Because findings for remission, response, and dimensional outcomes were similar, we only report on the results using remission as an outcome. We set the overall α value at 0.05, with the value for post-hoc pairwise comparisons between pairs of BMI classes at α=0.05/3=0.017.
Results
Prevalence of overweight and obesity
At baseline, slightly more than half (54.5%) of the participants were classified as normal, 48 (15.8%) were overweight, and 90 were obese (29.7%). Weight class membership was stable across time points; 90.5% of participants maintained assignment between baseline and week 12 (κ=0.90, 95% confidence interval [CI]: 0.85–0.95); 84.0% maintained assignment between baseline and week 24 (κ=0.82, 95% CI: 0.75–0.89).
Initial analysis of demographic and clinical correlates of overweight and obesity
Among the BMI groups, there were no differences with respect to sex (χ22=1.21, p=0.55), or race (χ22=1.23, p=0.54), but there were group differences for baseline age (F(2, 293)=6.16, p=0.002). Post-hoc contrasts showed that those in the obese group (15.4 years [SD=1.5]) were younger than those in the normal weight group (16.0 years [SD=1.5], p=0.008) or overweight group (16.2 years [SD=1.7], p=0.009) (Table 1).
Table 1.
Demographic and Clinical Characteristics by BMI Group
| Normal (n=158) | Overweight (n=48) | Obese (n=90) | Test | df | p | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age at baseline | 16.0±1.5a | 16.2±1.7a | 15.4±1.5b | F=6.16 | 2, 293 | 0.002 |
| Sex (n, % male) | 45 (28.5%) | 13 (27.1%) | 31 (34.4%) | χ2=1.21 | 2 | 0.55 |
| Race (n, % Caucasian) | 128 (81.6%) | 42 (87.5%) | 77 (85.6%) | χ2=1.23 | 2 | 0.54 |
| Depression | ||||||
| Number of MDD episodes | 1.3±0.6 | 1.3±0.7 | 1.4±0.6 | F=0.26 | 2, 286 | 0.77 |
| Duration of depression (months) | 21.9±19.8 | 28.6±25.4 | 22.2±20.1 | F=1.94 | 2, 287 | 0.15 |
| CDRS-R | 58.4±10.4 | 59.4±10.7 | 59.8±10.0 | F=0.55 | 2, 293 | 0.58 |
| BDI | 20.3±11.6 | 20.6±12.2 | 20.4±12.6 | F=0.01 | 2, 291 | 0.99 |
| Age at onset of current MDD | 14.1±2.1a | 13.6±2.6ab | 13.4±2.2b | F=2.88 | 2, 287 | 0.06 |
| Chronic depression (n, % Yes) | 91 (58.7) | 27(57.4) | 49 (54.4) | χ2=0.43 | 2 | 0.81 |
| Comorbidity | ||||||
| SCARED | 30.1±16.4 | 29.5±15.2 | 29.6±14.7 | F=0.06 | 2, 289 | 0.95 |
| History of PTSD (n, % Yes) | 13 (8.3) | 4 (8.3) | 5 (5.6) | FET | - | 0.79 |
| History of abuse (n, % Yes) | 43 (28.1)a | 13 (28.3)ab | 12 (13.6)b | χ2=7.10 | 2 | 0.03 |
| BHS | 10.1±5.2 | 10.5±5.9 | 10.6±5.8 | F=0.32 | 2, 286 | 0.73 |
| Functional status/Severity | ||||||
| CGAS | 50.6±7.8 | 50.9±8.8 | 50.6±7.2 | F=0.03 | 2, 291 | 0.98 |
| CGI-S | 4.5±0.7 | 4.5±0.7 | 4.4±0.6 | F=0.51 | 2, 292 | 0.60 |
| SIQ-Jr | 40.9±21.5 | 41.2±23.7 | 40.2±22.0 | F=0.04 | 2, 289 | 0.96 |
| Drug use | ||||||
| Drug use (n, % Yes) | 86 (55.1) | 25 (52.1) | 46 (51.7) | χ2=0.32 | 2 | 0.85 |
| Drug severity | 13.1±19.6a | 10.6±20.1ab | 6.5±14.5b | KWχ2=9.37 | 2 | 0.009 |
| Family conflict | ||||||
| CBQ-A | 9.4±6.3 | 7.7±5.6 | 8.5±6.3 | F=1.59 | 2, 287 | 0.21 |
| CBQ-P | 9.7±6.1 | 8.7±6.4 | 9.6±5.9 | F=0.46 | 2, 279 | 0.63 |
Data are mean±SD unless otherwise indicated.
Items with different superscripts are significantly different at α<0.017
BMI, body mass index; MDD, Major depressive disorder; PTSD, posttraumatic stress disorder; FET, Fisher's exact test; CDRS, Children's Depression Rating Scale (Poznanski et al, 1985); BDI, Beck Depression Inventory (Beck et al. 1988); SCARED, Self-Report for Childhood Anxiety Related Disorders (Birmaher et al. 1997); BHS, Beck Hopelessness Scale (Beck et al. 1974); CGAS, Children's Global Assessment Scale (Shaffer et al. 1983); CGI-S, Clinical Global Impressions-Severity subscale (Guy 1976); SIQ-Jr, Suicidal Ideation Questionnaire Junior High School version (Reynolds and Mazza 1999); CBQ-A, Conflict Behavior Questionnaire - Adolescent (Robin and Weiss 1980); CBQ-P, Conflict Behavior Questionnaire - Parent (Robin and Weiss 1980); KW: Kruskal-Wallis test.
Specific depressive symptoms at baseline (difficulty having fun, social withdrawal, sleep disturbance, appetite disturbance, and excessive fatigue) were not associated with BMI class (p's>0.18). The only other clinical correlate of BMI class was impairment related to drug and alcohol use on the DUSI (Kruskal-Wallis χ22=9.37, p=0.009) with pairwise contrasts indicating more severity in the normal weight than in the obese group (13.1 [SD=19.6] vs. 6.5 [SD=14.5], p=0.004).This result persisted after controlling for baseline age (relative risk ratio [RRR]=0.98, 95% CI: 0.96–1.00, z=−2.19, p=0.03). However, other clinical characteristics, such as duration of depression, number of episodes, and severity of depression (assessed via the CDRS-R) did not differ among the groups. There were group differences with respect to a history of abuse (χ22=7.10, p=0.03), with the normal weight group showing higher rates compared with the obese group (28.1% vs. 13.6%, p=0.001).
Multinomial logistic regression confirmed younger age (RRR=0.80, 95% CI: 0.66–0.94, z=−2.58, p=0.01), lower alcohol and drug related impairment (RRR=0.98, 95% CI: 0.96–1.00, z=−2.09, p=0.04), and history of abuse (RRR=0.46, 95% CI: 0.22–0.94, z=−2.13, p=0.03) as the most parsimonious set of correlates of obese as compared with the normal weight class. No correlates were significantly associated with normal weight compared with overweight (p's>0.21).
We also examined baseline correlates of change in BMI-z from intake to 24 weeks. Neither history of abuse, severity of depression (CDRS-R) nor age of onset of the depression significantly predicted growth in BMI-z (p's>0.32). Baseline age and number of MDD episodes were related to change in BMI-z (β=0.01, standard error [SE]=0.004, z=2.85, p=0.004 and β=−0.03, SE=0.009, z=−3.28, p=0.001 respectively).Among the symptoms of depression, there were no significant relationships between BMI-z growth and either fatigue, social withdrawal, increased appetite, or sleep disturbance (symptom by time interactions, p's>0.08). Similarly, baseline BMI, whether assessed continuously, or by weight category, was not related to BMI change over time (p's>0.08).
Treatment outcome by baseline BMI group
With respect to treatment course, rates of remission at 24 weeks were unrelated to BMI group both in univariate analyses (normal [38.0%], overweight [43.8%], obese [38.9%]; χ22=0.52, p=0.77), and after adjustment for baseline differences in age and drug and alcohol-related impairment (overweight vs. normal: odds ratio [OR]=1.15, 95% CI: 0.58–2.27, z=0.39, p=0.70; obese vs. normal: OR=0.88, 95% CI: 0.50–1.55, z=−0.45, p=0.65). We then tested whether BMI group moderated treatment outcome, by re-running the above analyses and also including medication and CBT terms, as well as interactions between BMI group and treatment type, and found no moderating effect of either medication (p=0.82) or CBT (p=0.64) on remission. These findings persisted when testing for moderation using the BMI-z score as a continuous measure, as compared with using categorical measures of BMI class (p's>0.73).
Mixed effects regressions were performed to determine if CDRS-R and BDI trajectories across 24 weeks differed among baseline BMI groups. The models included BMI group, time, and group by time interaction; whereas the effect of time was significant in both models (CDRS-R: β=−7.97, SE=0.35, z=−22.93, p<0.001. BDI: β=−3.55, SE=0.27, z=−13.31, p<0.001), there were no group by time interactions (p's=0.77 and 0.49 respectively).
In the subgroup (n=203), for whom serum drug concentration results were available, we found an association between baseline BMI status and serum drug level (p<0.001) (Sakolsky et al., 2011). Post-hoc pairwise comparisons showed that 69.1% of participants in the normal group versus 37.7% of participants in the obese group had week 6 serum levels at or above the geometric mean (χ21=15.86, p<0.001). Because we had previously found that treatment response was related to drug concentration and dosage in those treated with either citalopram or fluoxetine, we repeated these analyses in those treated with these two agents only, and also stratified these analyses by dosage, and still found no relationship between BMI class and outcome (p's>0.16) (Sakolsky et al. 2011).
BMI change as a function of treatment
Participants gained an average of 1.87 kg (SD=3.76, median=1.82, % change=2.69, 95% CI: 1.79–3.60) over 24 weeks, with corresponding change in BMI-z of 0.02 (SD=0.30, median=0.01). Significant weight gain (>7%) was experienced by 20.6% of the sample (SSRI: 23.0%, venlafaxine: 18.6%, χ21=0.38, p=0.54) (Table 2). The differential impact of medication type, CBT, and their interaction on BMI-z was examined using mixed effects regression. In these models, we controlled for baseline age and number of MDD episodes, because these variables were associated with change in BMI-z. There was no effect of CBT (p=0.75), but those who received an SSRI demonstrated a greater increase in BMI-z than did those given venlafaxine (β=−0.03, standard error [SE]=0.01, z=−2.45, p=0.01), with a weight gain of 2.34 kg (SD=3.97, median=2.27, % change=3.33, 95% CI: 1.90–4.76) in those treated with SSRIs versus 1.45 kg (SD=3.53, median=0.91, % change=2.13, 95% CI: 0.97–3.29) in those treated with venlafaxine and a corresponding mean change in BMI-z of 0.08 (SD=0.35, median=0.06) for SSRI, and −0.02 (SD=0.24, median=−0.02) for venlafaxine. We compared BMI-z changes among the three SSRIs. There was a group by time interaction (p=0.01). This effect was driven by an increase in BMI-z in those treated with paroxetine (β=0.05, SE=0.02, z=2.31, p=0.02) and citalopram (β=0.06, SE=0.02, z=2.68, p=0.007) as compared with those treated with fluoxetine. The corresponding change in weight was 2.82 kg (SD=4.90, median=3.86, % change: 4.21, 95% CI: 0.16–8.26) for paroxetine; 4.66 kg (SD=3.97, median=4.09, % change: 6.83, 95% CI: 3.86–9.80) for citalopram, and 1.05 kg (SD=2.98, median=1.82, % change: 1.31, 95% CI: −0.16–2.77) for fluoxetine. Also, the course of BMI-z in those treated with fluoxetine was not different than for those treated with venlafaxine (p=0.88).
Table 2.
Descriptive Statistics of Height, Weight and BMI
| |
Overall |
SSRI |
Venlafaxine |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 0 M (SD) | Week 12 M (SD) | Week 24 M (SD) | Week 0 M (SD) | Week 12 M (SD) | Week 24 M (SD) | Week 0 M (SD) | Week 12 M (SD) | Week 24 M (SD) | |
| n | 296 | 168 | 132 | 150 | 80 | 61 | 146 | 88 | 71 |
| Height (cm) | 164.6 (9.0) | 165.0 (8.3) | 165.6 (8.6) | 164.4 (9.4) | 164.5 (8.5) | 164.5 (7.9) | 164.8 (8.6) | 165.4 (8.1) | 166.4 (9.2) |
| Weight (kg) | 70.0 (19.7) | 71.7 (20.1) | 72.2 (20.2) | 70.3 (20.0) | 73.9 (21.6) | 73.7 (20.7) | 69.7 (19.4) | 69.6 (18.6) | 71.0 (19.9) |
| BMI | 25.7 (6.7) | 26.2 (6.7) | 26.3 (7.0) | 26.0 (6.9) | 27.2 (7.4) | 27.2 (7.8) | 25.5 (6.4) | 25.3 (6.0) | 25.5 (6.2) |
| BMI-z | 0.92 (1.0) | 1.01 (1.0) | 0.96 (1.1) | 0.94 (1.1) | 1.1 (1.1) | 1.0 (1.1) | 0.9 (1.0) | 0.9 (1.0) | 0.9 (1.0) |
BMI, body mass index; BMI-z, BMI-for-age-sex z-score; SSRI, selective serotonin reuptake inhibitor; M, mean.
Participants were allowed to enter the TORDIA study on a stable dose of stimulants (n=36, 10.9%). There was no difference in the change in BMI-z from baseline to 24 weeks between those treated with stimulants versus those not treated with stimulants (p=0.99).
We found no effect of SSRI versus venlafaxine on height over the course of the study (medication by time interaction, β=0.13, SE=0.13, z=1.04, p=0.30), and no change by type of SSRI (SSRI by time interaction, p=0.64; paroxetine vs. fluoxetine, β=0.17, SE=0.19, z=0.85, p=0.39; citalopram vs. fluoxetine, β=−0.02, SE=0.21, z=−0.07, p=0.94).
BMI change as a function of previous medication assignment
All participants in this study had been taking an SSRI prior to entry into the study. It is possible that the type of SSRI they were taking prior to entry into the study may have influenced their change in weight and BMI subsequent to a medication change. For example, the weight gain observed with paroxetine or citalopram could be explained in part as a return to the participants' pretreatment weight, after experiencing weight loss on fluoxetine. Conversely, the smaller amount of weight gain observed in those assigned to fluoxetine and venlafaxine in the trial could also have been influenced by weight loss after having been on citalopram, escitalopram, or paroxetine.
Therefore, we examined the course of BMI-z as a function of previous medication. Participants' previous medications were fluoxetine (n=92), paroxetine (n=41), sertraline (n=76), or escitalopram/citalopram (N=84); only three were taking fluvoxamine; therefore, these participants were not included in these analyses. We conducted a mixed-effects regression analysis with three dummy variables (paroxetine, sertraline, and fluoxetine) relative to citalopram/escitalopram, time, and their interaction, and found an overall previous treatment by time interaction (χ23=12.57, p=0.006), driven by a greater BMI-z growth in those previously treated with fluoxetine versus those previously taking citalopram/escitalopram (β=0.05, SE=0.02, z=3.12, p=0.002), and by a trend in those previously treated with fluoxetine versus those previously taking sertraline (β=0.03, SE=0.02, z=1.94, p=0.053). We then examined previous medication effect stratified by participant current assignment (i.e., citalopram, paroxetine, fluoxetine, or venlafaxine) and found a statistically significant relationship only in those randomized to venlafaxine (χ23=9.96, p=0.02), again driven by the contrast of fluoxetine versus citalopram/escitalopram (β=0.07, SE=0.02, z=3.15, p=0.002). In other words, previous treatment appeared to make a difference only in those who were randomized to venlafaxine; those switched from fluoxetine to venlafaxine showed a greater growth in BMI-z than did those switched from citalopram/escitalopram. In clinical terms, those venlafaxine-assigned participants switched from fluoxetine, compared with those switched from citalopram/escitalopram, had a greater growth in BMI-z (0.06 [SD=0.22] vs. −0.10 [SD=0.22]), and gained more weight (2.32 kg [SD=2.90] vs. −0.16kg [SD=3.47]).
BMI change as a function of remission
Mixed-effects regressions compared the change in BMI-z over time in those who responded or remitted versus those who did not; in these models we controlled for baseline age and number of MDD episodes. Change in BMI-z was unrelated to treatment response at 12 weeks (β=0.02, SE=0.01, z=1.38, p=0.17) or remission at 24 weeks (β=−0.004, SE=0.01, z=−0.34, p=0.74).
Discussion
In our sample of treatment-resistant depression, nearly 30% were classified as obese, which is double the prevalence in the general population. An early age of onset of depression was associated with a greater risk of obesity. BMI category was not predictive of treatment outcome, nor did it moderate treatment response. Conversely, the achievement of response or remission did not affect change in weight or BMI. However, there was a greater increase in BMI and weight in those treated with SSRIs as compared with those treated with venlafaxine. Post-hoc, these findings appear to have been driven by an increase in weight and BMI found in those treated with paroxetine or citalopram, relative to fluoxetine. Before placing these findings in the context of the extant literature, we first review the strengths and limitations of this study.
This study is relatively unique, insofar as there are no head-to-head comparisons of active treatments for adolescent depression that have examined changes in weight or BMI. One important limitation is that we do not have prior growth curve data, so that we cannot determine if the change in weight or BMI that occurred in this sample was a true change resulting from treatment, or a continuation of the baseline trajectory of weight change. The lack of a placebo condition makes it impossible to compare the natural change in weight over this period of time to any of our treatment conditions. Other limitations include the relatively short follow-up time, limited power to test within-SSRI differences in BMI change, and the post-hoc nature of the analyses. Wheras a post-hoc approach can be justified in a hypothesis-generating study, no corrections were made for the number of contrasts conducted, and as a result, these findings should be regarded cautiously. The number of contrasts may be the best explanation from some counterintuitive findings such as finding that both a history of abuse and of alcohol and substance use were less common in obese than normal weight individuals, counter to the literature (Felitti et al. 1998; Goldstein et al., 2008; McIntyre et al. 2012). Another limitation is that data were not gathered after a participant was determined to be a nonresponder, resulting in few data points for nonresponders compared with responders. However, we did not find a difference in the change in BMI as a function of either response or the number of assessments of height and weight; therefore, this is not likely to have biased the results. Finally, as this sample was a treatment-resistant group, many of whom had a history of chronic depression, these results cannot be generalized to acutely depressed adolescents being treated for the first time.
The high rate of obesity in this sample is likely related to the reported co-occurrence of depression and obesity. Supportive, although not proof-positive of a causal relationship between depression and obesity, is that the obese youth in this study were younger, and had an earlier initial occurrence of a depressive disorder. This suggests that earlier onset of depression may be a particularly salient risk factor for the development of obesity. This finding, coupled with other longitudinal studies that point to greater growth of BMI in those with depressive symptoms, indicates that early intervention for these youth are paramount to prevent the persistence of obesity into adulthood (Pine et al. 2001; Richardson et al. 2003). Some have reported greater increase in weight in antidepressant-treated patients who are of normal weight compared with those who are obese (Orzack et al. 1990), although we did not find that.
In this study, both citalopram and paroxetine were associated with significant weight increases, whereas venlafaxine and fluoxetine were not. The magnitude of weight increase, 2.8 and 4.7 kg for paroxetine and citalopram, respectively, is much larger than has been reported in previous adolescent depression trials. In an acute study of escitalopram, Emslie et al. (2009) found that both those on drug and on placebo gained on average 0.55 kg, whereas in an acute trial of sertraline in depressed children and adolescents, those treated with sertraline lost 0.23 kg, whereas those treated with placebo gained 0.78 kg (Wagner et al. 2006). Consistent with our findings, Nilsson et al. (2004) found relatively little change in weight on those treated with fluoxetine, with a smaller increase in both weight and height than in those treated with placebo: Height (fluoxetine, 1.0 cm [SD=2.4] vs. placebo, 2.1 cm [SD=2.6]; p=0.004) and weight (fluoxetine, 1.2 kg [SD=2.7] vs. placebo, 2.3 kg [SD=2.6]; p=0.008). However, the change in BMI-z, even in the citalopram/escitalopram group of 0.06 in 6 months, was modest compared with a change of 0.61 in the BMI-z in pediatric patients treated for nearly 2 years with risperidone (Calarge et al. 2012).
The difference in weight gain in our study versus these other studies may be explained by two factors: Our observation took place over a 24 week period rather than from 8 to 12 weeks, and this sample was treatment resistant and chronically depressed. Nevertheless, these findings suggest that, in treatment resistant, and probably all depressed youth, weight and BMI should be routinely monitored, and that in overweight youth, fluoxetine may be an agent associated with less weight gain. The association of paroxetine with weight gain is well established in the adult literature, although there is also evidence of an association of weight gain with the use of other antidepressants as well (Fava et al. 2000; Patten et al. 2009; Serretti and Mandelli 2010). Remarkably, none of the other child and adolescent clinical trials of the treatment of depression reported actual weight changes (Emslie et al. 1997; Keller et al. 2001; March et al. 2004; Emslie et al. 2006; Wagner et al. 2006).
One possibility we considered was that the weight changes that we saw on a given medication were more of a “rebound,” back to baseline, as compared with actual new weight gain. This turned out only to be the case in those who were treated with venlafaxine, who gained more weight if they had previously been treated with fluoxetine than if they had previously been treated with citalopram/escitalopram. This finding, however, is also consistent with the view that citalopram/escitalopram is associated with more weight gain than is fluoxetine.
Unlike some studies in adults, we did not find that higher BMI predicted a poorer response to antidepressants (Khan et al. 2007; Kloiber et al. 2007; Uher et al. 2009). In our protocol, we allowed for a dose increase, which may have prevented some participants with a larger volume of distribution caused by a higher BMI from having inadequate exposure to antidepressant. In fact, a lower geometric mean drug concentration was found in those with higher BMI. However, the absence of an association between BMI and medication treatment outcome in our sample may also be explained by the broad range of drug concentration values associated with an acceptable level of serotonin transport binding and treatment response (Meyer et al. 2004; Sakolsky et al. 2011). Given the association between obesity and lower drug concentration, and between lower drug concentration and outcome, further attention to the role of obesity in antidepressant response in adolescents is warranted despite these negative findings.
Conclusions
Our findings indicate that the treatment of depression is not sufficient to reverse obesity, as those youth whose depression remitted did not show a more favorable weight or BMI trajectory over 24 weeks. Therefore, although obese adolescent patients with depression may respond as well as nonobese patients to evidence-based treatments for depression, treatment of obesity requires additional interventions. However, feasibility studies in adolescents and adults suggest that aerobic exercise may be considered as either a stand-alone or an augmentation treatment for depression (Trivedi et al 2006; Dopp et al. 2012; Rimer et al.2012). Clinical trials in overweight and obese adolescents have shown that aerobic exercise, in addition to contributing to weight loss, also reduces depressive symptomatology, even in those with clinically significant depressive self-reported symptoms (Daley et al. 2006; Petty et al. 2009). A focus on increasing aerobic exercise could easily be incorporated into the behavior activation component of CBT.
Clinical Significance
In conclusion, we find that there is no short-term impact of remission of depression on BMI, nor of BMI on treatment response. This, in turn, suggests that one cannot assume that addressing weight problems will relieve depression or vice versa, but rather that each problem requires its own targeted intervention. It may be that depression may moderate the response to a weight reduction intervention, but as none was routinely offered in this study, we cannot comment on that possibility (Katon 2011). One clinically relevant finding was that increase in BMI and weight gain was greater in those treated with SSRIs, particularly paroxetine and citalopram, compared with those treated with either venlafaxine or fluoxetine. Therefore, fluoxetine may be preferable to citalopram, for example, in patients who are overweight, and conversely, patients treated with citalopram should have their weight and BMI carefully monitored. Given the high comorbidity between adolescent depression and obesity, clinicians treating adolescent depression should also assess BMI and collaborate with other healthcare professionals to insure optimal health and development for their depressed adolescent patients.
Acknowledgments
We thank study co-investigators: Betsy Kennard, Henrietta Leonard (deceased), Satish Iyengar, Lynn DeBar, Frances Lynch, James McCracken, Michael Strober, Robert Suddath, Benedetto Vitiello, and Robin Martin for manuscript preparation assistance; Mary Carter for editorial assistance; and the youth, families, and staff who made this project possible.
Disclosures
Dr. Emslie has received research support from BioMarin, Eli Lilly, Forest Laboratories, GlaxoSmithKline, and Somerset. He has served as a consultant for Biobehavioral Diagnostics, Eli Lilly, Forest Laboratories, GlaxoSmithKline, INC Research Inc., Lundbeck, Pfizer, Seaside Therapeutics, Shire, Valeant, and Wyeth Pharmaceuticals, and has been on the Speaker's Bureau for Forest Laboratories. Dr. Wagner has received honoraria from the American Psychiatric Association, American Academy of Child and Adolescent Psychiatry, UBM Medica, Quantia Communications, CME LLC, Nevada Psychiatric Association, Slack Inc, Mercy Hospital Universitario Ramón y Cajal, Las Vegas Psychiatric Society, and Partners Healthcare. Dr. Birmaher is currently employed by the University of Pittsburgh and the University of Pittsburgh Medical Center, Western Psychiatric Institute and Clinic, has received research funding from the NIMH, has or will receive royalties for publications from Random House, Inc. (New Hope for Children and Teens with Bipolar Disorder) and Lippincott Williams & Wilkins (Treating Child and Adolescent Depression). Dr. Brent is currently employed by the University of Pittsburgh, School of Medicine and the University of Pittsburgh Medical Center, Western Psychiatric Institute and Clinic, has received research support from the NIMH, receives royalties from Guilford Press, has or will receive royalties from the C-SSRS royalties from the electronic self-rated version of the C-SSRS from ERT, Inc., and serves as an UpToDate psychiatry editor. Messrs. Mansoor, Rengasamy, and Hilton; Mss. Porta, He, and Mayes; and Drs. Spirito, Clarke, Shamseddeen, and Ryan report no biomedical financial interests or potential conflicts of interest.
References
- American Psychiatric Association. 4th. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychiatric Association. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. [Google Scholar]
- Asarnow JR. Emslie G. Clarke G. Wagner KD. Spirito A. Vitiello B. Iyengar S. Shamseddeen W. Ritz L. McCracken J. Strober M. Suddath R. Leonard H. Porta G. Keller M. Brent D. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: predictors and moderators of treatment response. J Am Acad Child Adolesc Psychiatry. 2009;48:330–339. doi: 10.1097/CHI.0b013e3181977476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang KS. Chae SM. Hyun MS. Nam HK. Kim JS. Park KH. The mediating effects of perceived parental teasing on relations of body mass index to depression and self-perception of physical appearance and global self-worth in children. J Adv Nurs. 2012;68:2646–2653. doi: 10.1111/j.1365-2648.2012.05963.x. [DOI] [PubMed] [Google Scholar]
- Beck AT. Steer RA. Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Beck AT. Weissman A. Lester D. Trexler L. The measurement of pessimism: The Hopelessness Scale. J Consult Clin Psychol. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Khetarpal S. Brent D. Cully M. Balach L. Kaufman J. Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Boutelle KN. Hannan P. Fulkerson JA. Crow SJ. Stice E. Obesity as a prospective predictor of depression in adolescent females. Health Psychol. 2010;29:293–298. doi: 10.1037/a0018645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D. Emslie G. Clarke G. Wagner KD. Asarnow JR. Keller M. Vitiello B. Ritz L. Iyengar S. Abebe K. Birmaher B. Ryan N. Kennard B. Hughes C. DeBar L. McCracken J. Strober M. Suddath R. Spirito A. Leonard H. Melhem N. Porta G. Onorato M. Zelazny J. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA. Xie D. Fiedorowicz JG. Burns TL. Haynes WG. Rate of weight gain and cardiometabolic abnormalities in children and adolescents. J Pediatr. 2012;161:1010–1015. doi: 10.1016/j.jpeds.2012.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. A SAS program for the CDC growth charts. 2011. http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Apr 30;2012 ]. http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- Chapman DP. Perry GS. Strine TW. The vital link between chronic disease, depressive disorders. Prev Chronic Dis. 2005;2:A14. [PMC free article] [PubMed] [Google Scholar]
- Daley AJ. Copeland RJ. Wright NP. Roalfe A. Wales JK. Exercise therapy as a treatment for psychopathologic conditions in obese and morbidly obese adolescents: A randomized, controlled trial. Pediatrics. 2006;118:2126–2134. doi: 10.1542/peds.2006-1285. [DOI] [PubMed] [Google Scholar]
- Dopp RR. Mooney AJ. Armitage R. King C. Exercise for adolescents with depressive disorders: A feasibility study. Depress Res Treat. 2012;257472 doi: 10.1155/2012/257472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ. Mayes T. Porta G. Vitiello B. Clarke G. Wagner KD. Asarnow JR. Spirito A. Birmaher B. Ryan N. Kennard B. DeBar L. McCracken J. Strober M. Onorato M. Zelazny J. Keller M. Iyengar S. Brent D. Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. Am J Psychiatry. 2010;167:782–791. doi: 10.1176/appi.ajp.2010.09040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ. Rush AJ. Weinberg WA. Kowatch RA. Hughes CW. Carmody T. Rintelmann J. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry. 1997;54:1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Ventura D. Korotzer A. Tourkodimitris S. Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry. 2009;48:721–729. doi: 10.1097/CHI.0b013e3181a2b304. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Wagner KD. Kutcher S. Krulewicz S. Fong R. Carpenter DJ. Lipschitz A. Machin A. Wilkinson C. Paroxetine treatment in children and adolescents with major depressive disorder: a randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2006;45:709–719. doi: 10.1097/01.chi.0000214189.73240.63. [DOI] [PubMed] [Google Scholar]
- Fava M. Judge R. Hoog SL. Nilsson ME. Koke SC. Fluoxetine versus sertraline and paroxetine in major depressive disorder: Changes in weight with long-term treatment. J Clin Psychiatry. 2000;61:863–867. doi: 10.4088/jcp.v61n1109. [DOI] [PubMed] [Google Scholar]
- Felitti VJ. Anda RF. Nordenberg D. Williamson DF. Spitz AM. Edwards V. Koss MP. Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Gariepy G. Wang J. Lesage AD. Schmitz N. The longitudinal association from obesity to depression: Results from the 12-year National Population Health Survey. Obesity (Silver Spring) 2010;18:1033–1088. doi: 10.1038/oby.2009.333. [DOI] [PubMed] [Google Scholar]
- Goldstein BI. Strober MA. Birmaher B. Axelson DA. Esposito–Smythers C. Goldstein TR. Leonard H. Hunt J. Gill MK. Iyengar S. Grimm C. Yang M. Ryan ND. Keller MB. Substance use disorders among adolescents with bipolar spectrum disorders. Bipolar Disord. 2008;10:469–478. doi: 10.1111/j.1399-5618.2008.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. 2nd. Washington, DC: US Government Printing Office; 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- Hamer M. Batty GD. Kivimaki M. Risk of future depression in people who are obese but metabolically healthy: The English longitudinal study of ageing. Mol Psychiatry. 2012;17:940–945. doi: 10.1038/mp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain M. Victor RV. Hollett B. Weight gain and glucose dysregulation with second-generation antipsychotics and antidepressants: A review for primary care physicians. Postgrad Med. 2012;124:154–167. doi: 10.3810/pgm.2012.07.2577. [DOI] [PubMed] [Google Scholar]
- Hiemke C. Therapeutic drug monitoring in neuropsychopharmacology: Does it hold its promises? Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 1):21–27. doi: 10.1007/s00406-007-1005-y. [DOI] [PubMed] [Google Scholar]
- Keller MB. Lavori PW. Friedman B. Nielsen E. Endicott J. McDonald-Scott P. Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen. Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Keller MB. Ryan ND. Strober M. Klein RG. Kutcher SP. Birmaher B. Hagino OR. Koplewicz H. Carlson GA. Clarke GN. Emslie GJ. Feinberg D. Geller B. Kusumakar V. Papatheodorou G. Sack WH. Sweeney M. Wagner KD. Weller EB. Winters NC. Oakes R. McCafferty JP. Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2001;40:762–772. doi: 10.1097/00004583-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keller MB. Lavori PW. Friedman B. Nielsen E. Endicott J. McDonald–Scott P. Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Khan A. Schwartz KA. Kolts RL. Brown WA. BMI, sex, and antidepressant response. J Affect Disord. 2007;99:101–106. doi: 10.1016/j.jad.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Kirisci L. Mezzich A. Tarter R. Norms and sensitivity of the adolescent version of the Drug Use Screening Inventory. Addict Behav. 1995;20:149–157. doi: 10.1016/0306-4603(94)00058-1. [DOI] [PubMed] [Google Scholar]
- Kloiber S. Ising M. Reppermund S. Horstmann S. Dose T. Majer M. Zihl J. Pfister H. Unschuld PG. Holsboer F. Lucae S. Overweight and obesity affect treatment response in major depression. Biol Psychiatry. 2007;62:321–326. doi: 10.1016/j.biopsych.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Krebs NF. Himes JH. Jacobson D. Nicklas TA. Guilday P. Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM. Rohde P. Seeley JR. Major depressive disorder in older adolescents: Prevalence, risk factors, and clinical implications. Clin Psychol Rev. 1998;18:765–794. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- Libbey HP. Story MT. Neumark–Sztainer DR. Boutelle KN. Teasing, disordered eating behaviors, and psychological morbidities among overweight adolescents. Obesity (Silver Spring) 2008;16(Suppl 2):S24–29. doi: 10.1038/oby.2008.455. [DOI] [PubMed] [Google Scholar]
- March J. Silva S. Petrycki S. Curry J. Wells K. Fairbank J. Burns B. Domino M. McNulty S. Vitiello B. Severe J. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- McIntyre RS. Soczynska JK. Liauw SS. Woldeyohannes HO. Brietzke E. Nathanson J. Alsuwaidan M. Muzina DJ. Taylor VH. Cha DS. Kennedy SH. The association between childhood adversity and components of metabolic syndrome in adults with mood disorders: Results from the international mood disorders collaborative project. Int J Psychiatry Med. 2012;43:165–177. doi: 10.2190/PM.43.2.e. [DOI] [PubMed] [Google Scholar]
- Meyer JH. Wilson AA. Sagrati S. Hussey D. Carella A. Potter WZ. Ginovart N. Spencer EP. Cheok A. Houle S. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: An [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Moussavi S. Chatterji S. Verdes E. Tandon A. Patel V. Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Nanni V. Uher R. Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Nilsson M. Joliat MJ. Miner CM. Brown EB. Heiligenstein JH. Safety of subchronic treatment with fluoxetine for major depressive disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2004;14:412–417. doi: 10.1089/cap.2004.14.412. [DOI] [PubMed] [Google Scholar]
- Ostad Haji E. Hiemke C. Pfuhlmann B. Therapeutic drug monitoring for antidepressant drug treatment. Curr Pharm Des. 2012;18:5818–5827. doi: 10.2174/138161212803523699. [DOI] [PubMed] [Google Scholar]
- Orzack MH. Friedman LM. Marby DW. Weight changes on fluoxetine as a function of baseline weight in depressed adolescents. Psychopharmacol Bull. 1990;26:327–330. [PubMed] [Google Scholar]
- Patten SB. Williams JV. Lavorato DH. Eliasziw M. The effect of major depression on participation in preventive health care activities. BMC Public Health. 2009;9:87. doi: 10.1186/1471-2458-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty KH. Davis CL. Tkacz J. Young–Hyman D. Waller JL. Exercise effects on depressive symptoms and self-worth in overweight children: a randomized controlled trial. J Pediatr Psychol. 2009;34:929–939. doi: 10.1093/jpepsy/jsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Goldstein RB. Wolk S. Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- Poznanski EO. Freeman LN. Mokros HB. Children's Depression Rating Scale- Revised. Psychopharmacol Bull. 1985;21:979–989. [Google Scholar]
- Reynolds WM. Mazza JJ. Assessment of suicidal ideation in inner-city children and young adolescents: reliability and validity of the Suicidal Ideation Questionnaire-JR. School Psych Rev. 1999;28:17–30. [Google Scholar]
- Richardson LP. Davis R. Poulton R. McCauley E. Moffitt TE. Caspi A. Connell F. A longitudinal evaluation of adolescent depression and adult obesity. Arch Pediatr Adolesc Med. 2003;157:739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- Richardson LP. Garrison MM. Drangsholt M. Mancl L. LeResche L. Associations between depressive symptoms and obesity during puberty. Gen Hosp Psychiatry. 2006;28:313–320. doi: 10.1016/j.genhosppsych.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rimer J. Dwan K. Lawlor DA. Greig CA. McMurdo M. Morley W. Mead GE. Exercise for depression. Cochrane Database Syst Rev. 2012;7:CD004366. doi: 10.1002/14651858.CD004366.pub5. [DOI] [PubMed] [Google Scholar]
- Robin AL. Weiss JG. Criterion-related validity of behavioral and self-report measures of problem-solving communication skills in distressed and non-distressed parent-adolescent dyads. Behav Assess. 1980;2:339–352. [Google Scholar]
- Sakolsky DJ. Perel JM. Emslie GJ. Clarke GN. Wagner KD. Vitiello B. Keller MB. Birmaher B. Asarnow JR. Ryan ND. McCracken JT. Strober MJ. Iyengar S. Porta G. Brent DA. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31:92–97. doi: 10.1097/JCP.0b013e318204b117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A. Field AE. O'Reilly EJ. Fava M. Gortmaker S. Kawachi I. Ascherio A. Perceived and actual obesity in childhood and adolescence and risk of adult depression. J Epidemiol Community Health. 2013;67:81–86. doi: 10.1136/jech-2012-201435. [DOI] [PubMed] [Google Scholar]
- Serretti A. Mandelli L. Antidepressants and body weight: A comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- Shaffer D. Gould MS. Brasic J. Ambrosini P. Fisher P. Bird H. Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Simon GE. Von Korff M. Saunders K. Miglioretti DL. Crane PK. van Belle G. Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ. Faith MS. Allison KC. Depression and obesity. Biol Psychiatry. 2003;54:330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- Trivedi MH. Greer TL. Grannemann BD. Chambliss HO. Jordan AN. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12:205–213. doi: 10.1097/00131746-200607000-00002. [DOI] [PubMed] [Google Scholar]
- Uher R. Mors O. Hauser J. Rietschel M. Maier W. Kozel D. Henigsberg N. Souery D. Placentino A. Perroud N. Dernovsek MZ. Strohmaier J. Larsen ER. Zobel A. Leszczynska–Rodziewicz A. Kalember P. Pedrini L. Linotte S. Gunasinghe C. Aitchison KJ. McGuffin P. Farmer A. Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord. 2009;118:147–154. doi: 10.1016/j.jad.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Wagner KD. Jonas J. Findling RL. Ventura D. Saikali K. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry. 2006;45:280–288. doi: 10.1097/01.chi.0000192250.38400.9e. [DOI] [PubMed] [Google Scholar]
- Wang F. Wild TC. Kipp W. Kuhle S. Veugelers PJ. The influence of childhood obesity on the development of self-esteem. Health Rep. 2009;20:21–27. [PubMed] [Google Scholar]
- Young–Hyman D. Tanofsky–Kraff M. Yanovski SZ. Keil M. Cohen ML. Peyrot M. Yanovski JA. Psychological status and weight-related distress in overweight or at-risk-for-overweight children. Obesity (Silver Spring) 2006;14:2249–2258. doi: 10.1038/oby.2006.264. [DOI] [PMC free article] [PubMed] [Google Scholar]