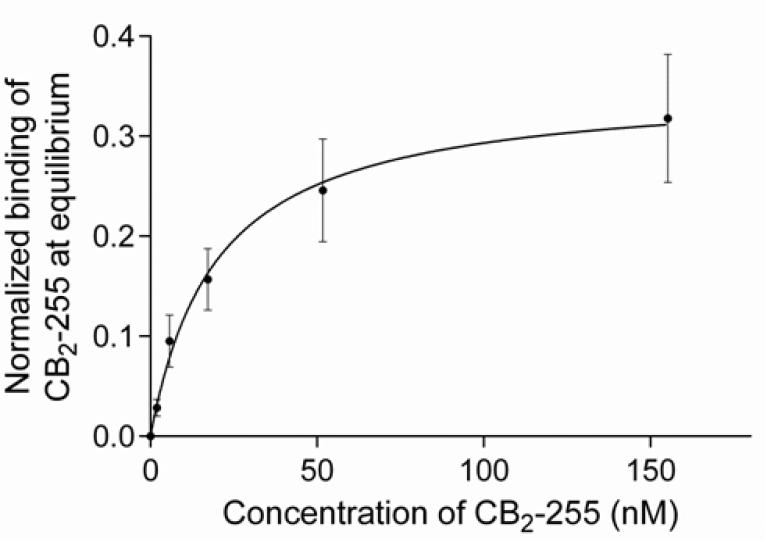
Figure 3. Thermodynamic characterization of the interaction between purified CB2 and the 1D4 antibody by SPR.
The normalized binding response of purified CB2-255 to 1D4 antibody-coated surfaces as a function of CB2 concentration is shown. 1D4 antibody was immobilized at three different surface densities while a reference flow cell was kept empty (see Materials and Methods). The steady-state responses from a stepwise titration for each CB2 concentration were determined at 1 hour (0, 1.9, 5.7 nM) and 40 minutes (17, 51, 155 nM) after injection at 5 μL/min. For normalization, the CB2-binding response was divided by the corresponding surface density RU-value of immobilized 1D4 antibody.
Steady state affinity analysis was performed using GraphPad Prism software. The value for KD (10 °C) of 19.9 ± 4.9 nM was obtained by non-linear regression analysis considering a 1:1 binding model.