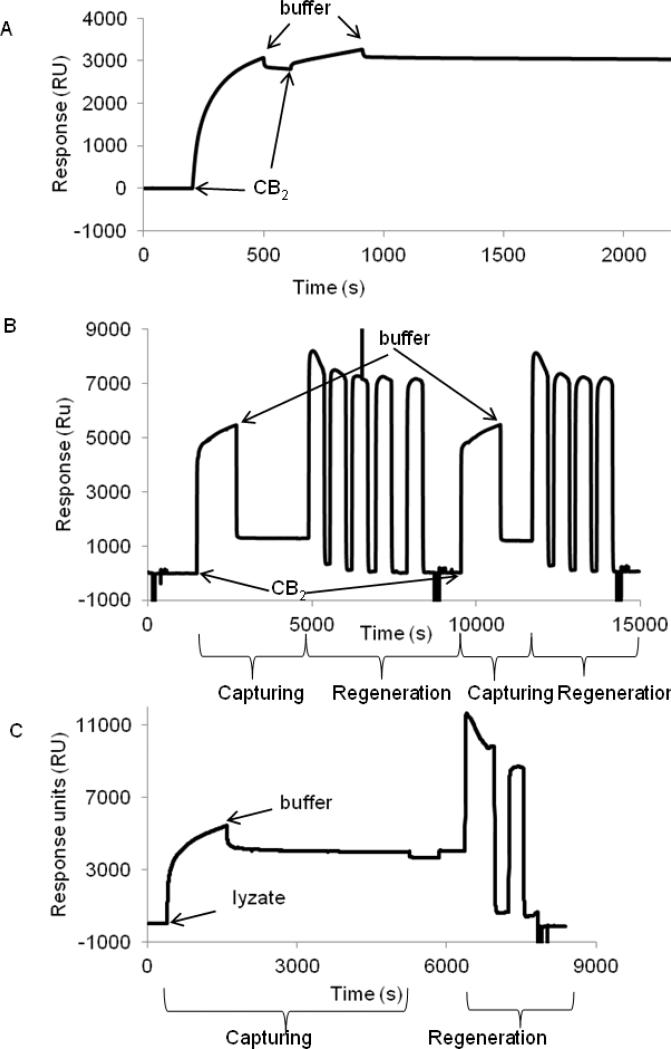
Figure 5. Capture of purified CB2 and fusion CB2-255 from crude lysate on a CM4 sensor chip surface.
A. Surface capture of CB2. Purified CB2-255 (0.5 μM) was injected at 2 μL/min for 10 min over empty (reference) - and 1D4 antibody-coated (~6000 RU) surfaces, followed by flow of running buffer (CSPR). The procedure was repeated for a second time. The experiment was carried out at 25 °C. The response from the reference surface was subtracted from the value of the experimental surface.
B. Regeneration of CB2-coated surface. Purified CB2-255 was captured on a 1D4 antibody-coated surface. For removal of CB2, a solution of Rho peptide (4 mM) in buffer CSPR supplemented with 1 M NaCl was injected four or five times slowly (1 μL/min) with contact times of 5-10 minutes each, followed by 1% OG in 10 mM NaOH two times at 50 μL/min for 5 sec each and buffer CSPR at the same flow rate for 1 min each. Two cycles of capturing and regeneration are shown.
C. Capture of fusion CB2 from cell lysate and regeneration of surface. Fusion protein was captured on a 1D4 antibody-coated surface by injecting the cell lysate at a rate of 2 μL/min for 20 min followed by injection of a running buffer.
For removal of captured protein, a solution of Rho peptide (4 mM) in buffer CSPR supplemented with 1 M NaCl was injected two times (1 μL/min) with contact times of 10, and 5 minutes each, followed by OG/NaOH and running buffer injections as described in B. The corresponding (not-referenced) sensorgram is shown.