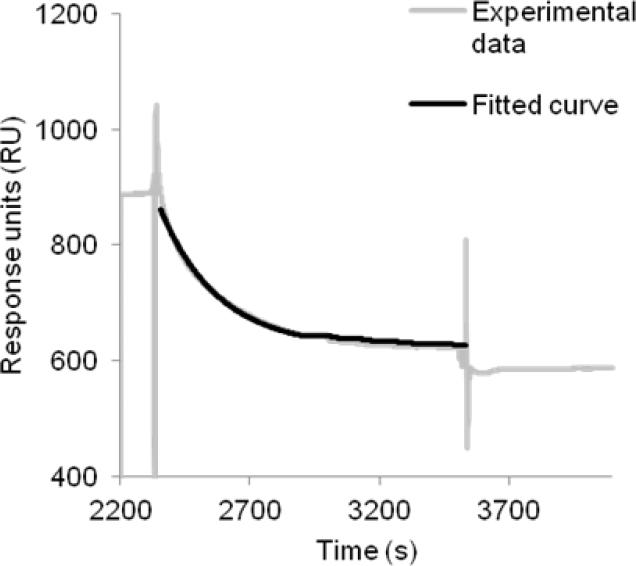
Figure 6. Enzymatic cleavage of fusion CB2-255 monitored by SPR.
The experiment was performed at 25 °C using buffer CSPR as a running buffer. After capture of fusion CB2 from crude extract to the high density 1D4 antibody-coated surface and removal of non-specifically retained material by injection of the running buffer, TEV protease (1 mg/mL in buffer CSPR) was injected at a flow rate of 2 μL/min for 20 min followed by injection of buffer for another 10 min. The specific response (after subtraction of a reference) as a function of time (dotted line) is shown. Data were fitted using an equation for exponential decay-one phase dissociation (black curve) using GraphPad Prism software. The rate constant for the enzymatic reaction was determined to be k (25 °C) = 4.5 × 10−3 s−1. The fit to the curve (solid line) is presented in the same graph.