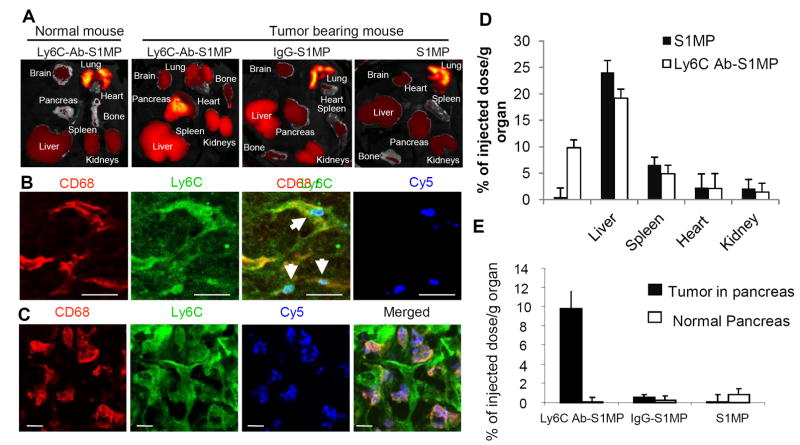
Figure 5.
Biodistribution and immunofluorescent analysis of the nanocarriers injected i.v. into the L3.6pl human tumor bearing or normal mice. (A): Ex vivo fluorescent imaging (IVIS) of the organs of normal mouse and the tumor bearing mice injected with the nanocarriers conjugated with Ly6C Ab (Ly6C-Ab-S1MP), control IgG (IgG-S1MP) or unconjugated nanocarriers (S1MP). (B and C): Immunofluorescent analysis of pancreatic tumors in the mouse injected with the nanocarriers conjugated with Ly6C Ab (labeled with Dylight 649 and detected through Cy5 channel). The nanocarriers attached to endothelial cells (CD31) in capillaries which also expressed Ly6C (emitted yellow fluorescence) 15 minutes after i.v. injection (indicated by white arrows). The nanocarriers were further engulfed by CD68 and Ly6C positive tumor associated macrophages as identified 4 hours after the injection. These results are representative of two independent experiments. Bar=5μm; (D) ICP-AES quantitative analysis of targeted (Ly6Cab-S1MP, white) and untargeted (SMP, black) nanocarriers biodistribution in various organs (n=4-5); (E) ICP-AES quantitative analysis of nanocarriers biodistribution revealed that 9.8±2.3% of injected dose/g tumor of the Ly6C-Ab-S1MP accumulated in the pancreatic tumors as opposed to 0.5±1.8% with IgG-S1MP (n=4-5). In normal pancreas, accumulation of S1MP was low, regardless of the surface modification.