Abstract
Objective
The primary aims of this study were to 1) determine whether folate metabolism genetic polymorphisms predict age of onset and occurrence of late life depression and 2) determine whether folate metabolism genetic polymorphisms predict response to antidepressant medications in late-life depression.
Methods
This study used the Conte Center for the Neuroscience of Depression, and the Neurocognitive Outcomes of Depression in the Elderly Study database which includes individuals age ≥ 60. The folate nutrition assessment was determined by the Block Food Frequency Questionnaire. Genotype was evaluated for 15 single nucleotide polymorphisms (SNPs) from 10 folate metabolism genes. Logistic regression models were used to examine genetic polymorphisms and folate estimates with association with depression age of onset and remission status.
Results
There were 304 Caucasians in the database, 106 of these who were not depressed and 198 who had a diagnosis of depression. There were no significant differences between remitters and nonremitters in age, sex or estimated folate intakes. There were no folate estimates or folate metabolism gene SNPs that significantly predicted age of onset of depression or occurrence of depression. MTRR A66G (rs1801394) was significantly associated with remission status (p=0.0077) such that those with the AA genotype were 3.2 times as likely as those with the GG genotype to be in remission (p=0.0020). MTHFR A1298C (rs1801131) achieved a borderline significance for association with remission status (p=0.0313).
Conclusion
The major finding from this study is that the MTRR A66G genotype predicts response to SSRI antidepressants in late life depression.
Keywords: antidepressants, depression, late-life depression, depressive disorder/genetics, single nucleotide polymorphisms, folate
INTRODUCTION
Depression occurs in approximately 5 to 10% of community dwelling elderly persons (Blazer 1989). Approximately 50–75% of elderly depressed patients continue to be depressed despite receiving appropriate treatment (Unutzer 2002, Trivedi 2006). An emerging body of evidence suggests that vitamin and nutritional factors may play a role in treatment outcomes and may explain depression occurrence and lack of response to antidepressant treatment (Morris 2003, Papakostas 2004). The role of nutrition is particularly relevant for the older depressed patient, as this population is at significant risk for under-nutrition. Specifically, in one community-based study, one fourth of elderly persons were at high risk for malnutrition (Vailas 1995). Malnutrition may be associated with insufficient folate intake or metabolism. These folate inadequacies may in turn influence depression via brain mechanisms including serotonin synthesis and release, myelin synthesis, or by influencing homocysteine metabolism which may affect both brain and vascular health (Paul 2004).
Low folate levels can occur in up to one-third of depressed patients and are associated with depression risk and resistance to antidepressant treatment in geriatric patients (Alpert 2003. Gilbody 2007a, Kim 2008). In older adults, dietary studies have shown that intake of food folate is lower in older adults with depression than in non-depressed individuals (Kim 2008, Payne 2009), although some studies have found no association (Kamphuis 2008, Skarupski 2010). Depressed patients with low or deficient serum folate have also been shown to have a lower response to a selective serotonin reuptake inhibitor (SSRI) antidepressant (Papakostas 2004, 2005, Fava 1997) and a higher response to antidepressant therapy when folic acid was supplemented (Coppen 2000).
The folate cycle is a highly complex process which is crucial to the transfer of one carbon units for nucleic acid and amino acid synthesis (Figure 1). The folate cycle and methionine cycle are linked via the conversion of homocysteine to methionine. Genetic factors related to folate metabolism have been implicated in depression occurrence. Methylenetetrahydrofolate reductase (MTHFR) irreversibly converts 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the rate limiting step of folate metabolism. The MTHFR C677T polymorphism is a common genetic variant resulting in an alanine to valine substitution. Individuals homozygous for the T allele (TT) as well as heterozygous carriers (TC) are more likely to be depressed as compared with individuals with the CC genotype for this polymorphism. (Arimini 1997, Bjelland 2003, Lewis 2006, Gilbody 2007b). However, there has been much debate as to whether this MTHFR genotype in isolation is associated with depression symptoms. In a meta-analysis that limited inclusion to only case control studies with populations of European ancestry, no association was found between 677TT genotype or T allele and depression (Gaysina 2008). Although not as widely studied, the MTHFR A1298C polymorphism was associated with depression in a population of women of European descent (Reif 2005). In addition to these MTHFR polymorphisms, there are several other single nucleotide polymorphisms (SNPs) in the folate metabolic pathway that have been identified (Boyles 2006), but their role and function in depression etiology and treatment are presently not known.
Figure 1.
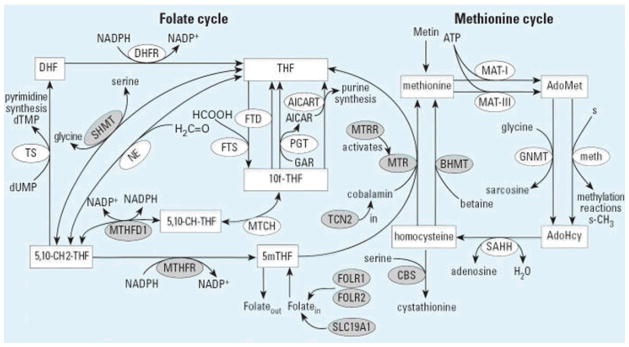
The folate and methionine cycles including the 10 genes evaluated in this study. Substrates are shown in rectangular boxes; enzymes are shown in ellipses. Boyles 2006
In order to broaden the understanding of the relationships between folate intake, folate metabolism genes and depression outcomes, we evaluated 10 of these genes with the goal to 1) determine whether folate metabolism genetic polymorphisms predict age of onset and occurrence of late life depression and 2) determine whether folate metabolism genetic polymorphisms predict response to antidepressant medications in late-life depression and 3) determine whether there is an interaction between folate status and folate metabolism genes in predicting antidepressant response in late-life depression. We hypothesized that we would identify folate metabolism genes associated with occurrence of depression, age of onset of depression and response to antidepressant treatment. We further hypothesized that genetic associations with antidepressant treatment, if present, would be modified by folate status.
METHODS
Data set description
This study used the National Institute of Mental Health supported Conte Center for the Neuroscience of Depression, and the Neurocognitive Outcomes of Depression in the Elderly Study database. Comparison participants were recruited from the community and the Aging Center Subject Registry at Duke University. The cross sectional analysis evaluated age of onset of depression and depression occurrence as dependent variables. The longitudinal analysis evaluated antidepressant treatment response as the dependent variable. The study was approved by the Duke Institutional Review Board. After explanation of the study, patients who provided informed consent were enrolled in the study. A separate consent was used for genetic sampling.
The inclusion criteria for the depression study were 1) Age ≥ 60 years, (2) Major depression, single episode or recurrent diagnosed by DSM-IV criteria (3) Ability to read and write English, (4) Mini-Mental State Examination (MMSE) score ≥25 (rule out dementia), (5) Willingness to participate in the follow-up study for at least two years. The exclusion criteria were (1) lifetime alcohol or drug dependence, (2) conditions associated with MRI abnormalities such hydrocephalus, benign and cancerous brain tumors, epilepsy, Parkinson’s disease, Huntington’s chorea, dementia, demyelinating diseases, etc., (3) endocrine disorder other than diabetes mellitus (4) any physical or intellectual disability that may affect completion of self rating instruments, (5) established clinical diagnosis of dementia, (6) Other primary psychiatric disorders, including panic disorder, social phobia, non-affective psychosis (including schizo-affective disorder), schizophrenia, bipolar disorder (7) Any metal or pacemaker in the body which precluded MRI.
A study geriatric psychiatrist treated patients using a guideline-based somatic treatment approach and assessed depression severity using the Montgomery-Asberg Depression Rating Scale (MADRS) as previously described (Steffens 2002). The MADRS was repeated a minimum of every 3 months to monitor treatment response. At enrollment, demographic information was collected (age, sex, age at depression diagnosis, race). In addition to examining subjects taking nonSSRIs, we also examined the subgroup of subjects who were prescribed SSRI antidepressant medications as monotherapy (i.e., paroxetine, citalopram, fluoxetine, escitalopram, sertraline). Treatment response was measured as remission (MADRS < 8), response (MADRS end 8–15 inclusive) or nonresponse (MADRS end >15) over the acute 12 week treatment period. Only Caucasian subjects were used for this genetic analysis due to anticipated population differences between ethnic groups in allele frequencies.
Genotyping
Depression occurrence, age of onset of depression and treatment response by genotype was evaluated for 15 single nucleotide polymorphisms (SNPs) from 10 folate metabolism genes (Table 1). We examined SNPs for ten genes of the folate pathway by a method previously described (Boyles 2006). All but two genetic variants were genotyped by commercially available TaqMan allelic discrimination assays (Assays-on-Demand and Assays-by-Design, Applied Biosystems, Foster City, CA). Previously published polymerase chain reaction (PCR) primers for a 68-bp insertion in CBS exon 8 (Morrison 1998) produced results that did not pass quality control measures. Sequencing of the insertion showed a tandem duplication such that the forward primer hybridized before and within the insertion. We used a forward primer 58 base pairs further upstream of the insertion producing 242 or 310 bases fragments (forward, 5′-CGGCGGTATTGGCCACTC-3′; reverse, 5′-GGCCGGGCTCTGGACTC-3′). All PCR amplification used the GeneAmp PCR system 9700 thermocyclers (Applied Biosystems) according to assay specifications. Fluorescence was detected with the ABI Prism 7900HT Sequence Detection System and analyzed with ABI Prism Sequence Detection System software (version 2.0; Applied Biosystems). All assays included blinded duplicate samples, as well as two CEPH samples, and were required to meet a minimum of 95% efficiency (i.e. 95% or more of the genotypes had to be called with certainty).
Table 1.
Folate SNPs Genotyped
| Gene symbol (with name) | SNP | Alleles | Minor Allele | Prevalence of Genotypes in Study Caucasian Population |
|---|---|---|---|---|
| MTHFR (methylene tetrahydrofolate reductase) | RS1801131 | A/C | C | AA- 142/302 (47.02%) AC- 133/302 (44.04%) CC- 27/302 (8.94%) |
| MTHFR (methylene tetrahydrofolate reductase) | RS1801133 | C/T | T | CC- 125/304 (41.12%) CT- 141/304 (46.38%) TT- 38/304 (12.5%) |
| MTR (5-methyltetrahydrofolate-homocysteine methyltransferase) | RS1805087 | A/G | G | AA- 193/297 (64.98%) AG- 95/297 (31.99%) GG- 9/297 (3.03%) |
| MTRR (5-methyltetrahydrofolate-homocysteine methyltransferase reductase) | RS1801394 | A/G | G | AA- 74/301 (24.58%) AG- 147/301 (48.84%) GG- 80/301 (26.58%) |
| BHMT (betaine-homocysteine methyltransferase) | RS3733890 | C/T | T | CC- 143/301 (47.51%) CT- 131/301 (43.52%) TT- 27/301 (8.97%) |
| FOLR1 (folate receptor 1) | RS2071010 | A/G | A | AA- 0 AG- 35/296 (11.82%) GG- 261/296 (88.18%) |
| FOLR2 (folate receptor 2) | RS2298444 | C/T | C | CC- 10/301 (3.32%) CT- 81/301 (26.91%) TT- 210/301 (69.77%) |
| MTHFD1 (methylenetetrahydrofola te dehydrogenase 1) | RS2236225 | A/G | A | AA- 72/299 (24.08%) AG- 145/299 (48.49%) GG- 82/299 (27.42%) |
| SHMT1 (serine hydroxy-methyltranferase 1) | RS1979277 | C/T | T | CC- 130/297 (43.77%) CT- 132/297 (44.44%) TT- 35/297 (11.78%) |
| CBS (cystathionine-beta-synthase) | RS412810 | A/C | C | AA- 153/297 (51.52%) AC- 112/297 (37.71%) CC- 32/297 (10.77%) |
| CBS (cystathionine-beta-synthase) | RS1801181 | A/G | A | AA- 44/297 (14.81%) AG- 129/297 (43.43%) GG- 124/297 (41.75%) |
| CBS (cystathionine-beta-synthase) | RS4920037 | A/G | A | AA- 23/300 (7.67%) AG- 92/300 (30.67%) GG- 185/300 (61.67%) |
| CBS (cystathionine-beta-synthase) | RS234715 | G/T | T | GG- 181/297 (60.94%) GT- 97/297 (32.66%) GG- 19/297 (6.4%) |
| CBS (cystathionine-beta-synthase) | RS234783 | C/T | C | CC- 76/296 (25.68%) CT- 145/296 (48.99%) TT- 75/296 (25.34%) |
| TCN2 (transcobalamin II) | RS18011198 | C/G | G | CC- 95/298 (31.88%) CG- 149/298 (50%) GG- 54/298 (18.12%) |
Hardy-Weinberg Equilibrium (HWE) was evaluated with exact tests implemented in Genetic Data Analysis (Zaykin 1995). Only 1 SNP (rs4920037) in CBS deviated from HWE (p=0.0091). Linkage disequilibrium (LD) was computed using GOLD (Abecasis 2000).
Dietary Assessment
The nutrition assessment, a Block 1998 Food Frequency Questionnaire (FFQ), and protocol have been described previously (Payne 2006). Depressed individuals were not given an assessment when they were severely depressed because of concerns about cognitive impairment and subject burden, so that in most cases dietary assessment post-dated the baseline assessment.
Intake estimates included total kilocalories and folate. Folate estimates included dietary folate equivalents (DFEs) for natural food folate, folic acid from fortified foods, and folic acid from supplements. DFEs were used instead of μg to account for increased bioavailability of the folic acid form [DFE = folate (μg) + [1•7 * folic acid (μg)] (Dietary References 1998).
Statistical Analysis
General descriptive statistics and regression models were run using SAS software version 9.2 (SAS Institute Inc., Cary, NC). Contingency tables (PROC FREQ) and means (PROC MEANS) were generated to evaluate baseline characteristics of the remitter and nonremitter groups.
Several logistic regression models were examined. Folate estimates were tested for association with depression age of onset (covariates included sex and calories) as well as with remission status (remission, response, no response; covariates included sex, calories, and age at enrollment). Genetic polymorphisms were also tested for association with depression age of onset (controlling for sex) and remission status (controlling for sex, and age at enrollment).
To control for multiple testing, we utilized SNP spectral decomposition (Li and Ji 2005) to determine the effective number of independent statistical tests based on LD among SNPs in the same gene. The adjusted p-value threshold for significance was p=0.0033.
RESULTS
Of the 336 Caucasian subjects in our dataset, 304 had genetic data available and were eligible for the analysis. Of the198 subjects with a diagnosis of depression, 119 were prescribed SSRI antidepressants while 104 also had complete folate nutrition data.
Cross-sectional results
See Table 2 for descriptive statistics. There were no significant differences between remitters and nonremitters in age, sex or estimated folate intakes. Remitters had a higher age of onset than non-remitters (p=0.01). There were no folate estimates or SNPs that significantly predicted age of onset of depression or occurrence of depression.
Table 2.
Demographics, nutrition and clinical characteristics
| Variable | Overall | Remitters (MADRS_END < 8) | Non-Remitters (MADRS_END ≥ 8) | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | mean (%) | std dev | N | mean (%) | std dev | N | mean (%) | std dev | ||
| age at enrollment | 104 | 67.6 | 6.6 | 55 | 67.25 | 6.0 | 49 | 68.0 | 7.2 | 0.5580 |
| sex (% Male) | 104 | 42.3% | 55 | 38.2% | 49 | 46.9% | 0.4285 | |||
| age of onset | 104 | 42.6 | 20.3 | 55 | 47.4 | 18.3 | 49 | 37.39 | 21.19 | 0.0110 |
| MADRS score at enrollment | 104 | 24.9 | 6.7 | 55 | 25.1 | 7.3 | 49 | 24.6 | 6.1 | 0.6546 |
| Dietary Folate* | ||||||||||
| Natural food folate | 104 | 218.9 | 84.8 | 55 | 220.8 | 84.3 | 49 | 216.9 | 86.1 | 0.8172 |
| Fortified folate | 104 | 139.3 | 95.3 | 55 | 135.2 | 83.2 | 49 | 143.9 | 108.1 | 0.6456 |
| Total food folate | 104 | 455.8 | 215.8 | 55 | 450.7 | 191.3 | 49 | 461.5 | 242.4 | 0.7992 |
| Supplemental folate | 104 | 514.7 | 494.6 | 55 | 462.7 | 441.2 | 49 | 572.9 | 547.1 | 0.2587 |
| Total Folate | 102 | 949.5 | 520.4 | 55 | 922.2 | 506.4 | 47 | 981.5 | 539.9 | 0.5683 |
DFE= Dietary Folate Equivalents
Longitudinal Results
Genotype and Antidepressant Response
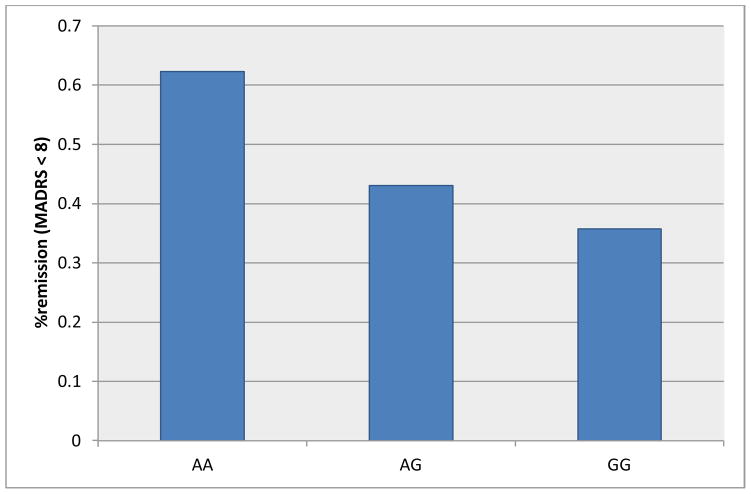
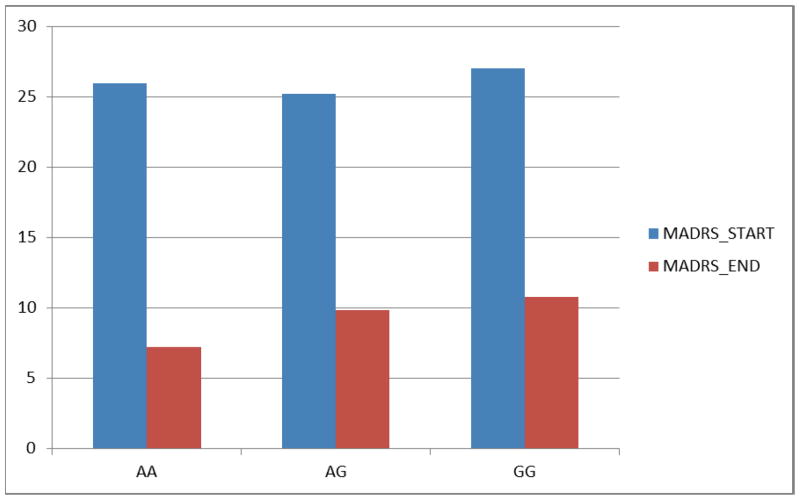
Significant associations between SNPs and remission status are shown in Table 3. MTRR A66G (rs1801394) was significantly associated with remission status (p=0.0077) such that those with the AA genotype were 3.2 times as likely as those with the GG genotype to be in remission (p=0.0020). Likewise, those with the AG genotype were 2.1 times as likely as those with the GG genotype to be in remission (p=0.0278). This association was even more significant among those subjects taking SSRI antidepressants (p=0.0060) (Figure 2). The proportion of patients achieving remission was consistent with the 12 week mean MADRS change from baseline scores among the SSRI antidepressant subjects (Figure 3).
Table 3.
SNPs and remission status in subjects taking SSRIs
| SNP | comparison | OR | CI | comparison p-value* | global p-value* |
|---|---|---|---|---|---|
| MTRR A66G (RS1801394) | 0.0077 | ||||
| CC vs TT | 0.3097 | 0.1472, 0.6516 | 0.002 | ||
| CT vs TT | 0.4673 | 0.2373, 0.9203 | 0.0278 | ||
| CC vs CT | 0.6628 | 0.3507, 1.2526 | 0.2054 | ||
| MTHFR A1298C (RS1801131) | 0.0313 | ||||
| AA vs CC | 1.1371 | 0.3425, 3.7748 | 0.8337 | ||
| AC vs CC | 2.8828 | 0.8472, 9.8088 | 0.0901 | ||
| AA vs AC | 0.3945 | 0.1882, 0.8265 | 0.0137 |
unadjusted p value. The adjusted p-value threshold for significance was p=0.0033.
Figure 2.
Proportion of patients taking SSRI antidepressants who achieved remission by the 12 week assessment by MTRR A66G (rs1801384) genotype where remission was defined as MADRS <8; overall p-value = 0.006. The adjusted p-value threshold for significance was p=0.0033
Figure 3.
Baseline start and end of 12 week treatment Mean MADRS values for patients taking SSRI antidepressants by MTRR A66G (rs1801384) genotype.
Among those taking SSRI antidepressants, MTHFR A1298C (rs1801131) achieved only a borderline significant association with remission status when taking into account the threshold for multiple comparisons (p=0.0313). Those subjects with the AC genotype were 2.5 times as likely as those with the AA genotype to be in remission (p=0.0137).
Folate by Genotype Interaction
Following the univariate genotype analysis, we explored possible gene-folate interactions for antidepressant response. . Although there was a marginally significant SNP by folate interaction for MTHFR A1298C, when examining likelihood ratios there were no robust associations of gene by folate status to report.
DISCUSSION
This study examined the association of late-life depression occurrence, age of onset of depression and antidepressant response with the spectrum of folate metabolism genes. The major finding from this study is that the MTRR A66G genotype predicts response to SSRI antidepressants in late life depression in a US caucasian population. Specifically individuals who are homozygous at the G allele are less likely to be in remission after a course of SSRI antidepressant treatment compared to individuals who are homozygous at the A allele or who are AG heterozygotes. With regard to MTHFR A1298C, subjects with the AA genotype are less likely to be in remission as compared to those with the AC genotype. To our knowledge, this is the first study to report MTRR A66G and MTHFR A1298C polymorphisms to be associated with SSRI antidepressant response. As previous genetic association studies in the depression literature have focused primarily on serotonin related genes, this study significantly broadens our understanding of other pathways and significantly adds to the genetic literature on depression and antidepressant treatment.
Methionine synthase reductase (MTRR) is a key regulatory enzyme required to restore methionine synthase (MTR) in the homocysteine metabolic pathway. MTR catalyzes transmethylation of homocysteine to methionine in a cobalamin dependent reaction that utilizes methyltetrahydrofolate as a methyl donor. The common variant of the MTRR gene (66A→G) replaces methionine for isoleucine and appears to result in reduced activity for restoring methionine synthase (Olteanu 2001). Previous studies have shown that the GG polymorphism has been associated with an increased risk for neuronal tube defects (Zhu 2003), and congenital heart defects (Zeng 2011). The finding from our study that GG homozygotes were less likely to be in remission also points toward the hypothesis that factors which elevate homocysteine concentrations have an adverse effect on depression treatment response (Coppen 2000).
Methylenetetrahydrofolate reductase (MTHFR) C677T results in reduced enzyme activated (approximately 40% of control) and hyperhomocystenemia (Lievers 2001). In a recent study examining response to citalopram in subjects with depression secondary to traumatic brain injury, 677T predicted a lower treatment response (Lancot 2010). A second MTHFR polymorphism A1298C also has a significant effect on enzyme activity, but was not associated with increased homocysteine concentrations (Lievers 2001). The 1298C variant has been associated with major depression in one small study of middle aged women of German ancestry (Reif 2005) with heterozygotes having the highest rate of major depression diagnosis. In our study, individuals who were heterozygotes were also more likely to be in remission, however, given that the statistical association was borderline when accounting for multiple statistical testing, this finding must be replicated before any conclusions can be drawn.
The strengths of our study are that we were able to evaluate a large spectrum of folate metabolizing genes, some of which have not been examined previously. Previous studies have focused on whether folate status is associated with antidepressant response or whether MTHFR variants are associated with depression occurrence. In our study, we analyzed both genetic and folate determinants and were able to evaluate if an interaction existed between these two factors. In addition due to the higher prevalence of low folate levels in the elderly, the population of older adults that we studied with late life depression are those that may be more at risk for poorer outcomes related to diet and folate specific genetic determinants.
Limitations of our study include the small sample size which resulted in limited power to identify genotype associations. Our study design was based on a retrospective analysis of antidepressant treatment response. The choice of antidepressant treatment was based on clinical criteria and may have been associated with past success with certain antidepressants and tolerance to medication. We chose a MADRS cutoff of < 8 as our primary assessment of antidepressant efficacy. An equally valid choice may have been the use of a MADRS cutoff of < 15 which has been associated with antidepressant response. However, we chose the more conservative MADRS cutoff value of < 8 as it more closely aligns with an assessment of remission as determined by the clinical global impression – severity (CGI-S) value of 1 (normal) (Hawley 2002).
The dietary folate assessment is subject to recall bias and was not performed until post-baseline, making interpretation problematic. In addition, there is only a modest correlation between dietary folate estimates and serum folate. However, food frequency estimates have been shown to successfully rank participants along the folate intake spectrum (Iso 2003, Signorello 2010). Finally, we did not have serum measures of folate status which would have been helpful for interpretation of the relationship between diet, folate metabolism genes and depression.
To our knowledge, this is the first study to report an association between polymorphism at MTRR A66G and SSRI antidepressant response. Given that this study is the first to demonstrate treatment associations with this folate metabolism gene, further confirmation of these findings in a larger sample is warranted as well as determination of whether this finding is consistent in a noncaucasian population.
Key Points.
Folate metabolism genes may play a key role in response to antidepressants.
The MTRR A66G genotype predicts response to SSRI antidepressants in late life depression in a US caucasian population.
Further confirmation of these findings in a larger sample is warranted as well as determination of whether this finding is consistent in a noncaucasian population.
Acknowledgments
Sources of Support: This project was funded by National Institutes of Health grants MH40159, MH54846, MH60451, MH70027, and ES011961 (Environment Health Sciences Pilot Center Grant).
References
- Abecasis GR, Cookson WO. GOLD--graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–3. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- Alpert M, Silva R, Pouget E. Prediction of Treatment Response in Geriatric Depression from Baseline Folate Level: Interaction with an SSRI or a Tricyclic Antidepressant. J Clin Psychopharm. 2003;23(3):309–313. doi: 10.1097/01.jcp.0000084024.22282.cd. [DOI] [PubMed] [Google Scholar]
- Arinami T, Yamada N, Yamakawa-Kobayashi K, Hamaguchi H, Toru M. Methylenetetrahydrofolate reductase variant and schizophrenia/depression. American Journal of Medical Genetics. 1997;74:526–528. doi: 10.1002/(sici)1096-8628(19970919)74:5<526::aid-ajmg14>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Belousov YS, Welch RA, Sanders S, Mills A, Kulchenko A, Dempcy R, et al. Single nucleotide polymorphism genotyping by two colour melting curve analysis using the MGB Eclipse Probe System in challenging sequence environment. Hum Genomics. 2004;1:209–217. doi: 10.1186/1479-7364-1-3-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelland I, Tell G, Vollset SE, Refsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C→T polymorphism in anxiety and depression. Archives of General Psychiatry. 2003;60:618–626. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- Blazer D. Depression in the elderly. N Engl J Med. 1989;320:164–6. doi: 10.1056/NEJM198901193200306. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T. Homocysteine and folate metabolism in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(7):1103–12. doi: 10.1016/j.pnpbp.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Boyles AL, Billups AV, Deak MC, Siegel DG, Mehltretter L, Silfer H, Bassuk AG, Kessler JA, Reed MC, Nijhout F, George TM, Enterline DS, Gilbert JR, Speer MC the NTD Collaborative Group. Neural Tube Defects and Folate Pathway Genes: Family-Based Association Tests of Gene–Gene and Gene–Environment Interactions. Environ Health Perspect. 2006;114:1547–1552. doi: 10.1289/ehp.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomized, placebo-controlled trial. J of Affective Disorders. 2000;60:121–130. doi: 10.1016/s0165-0327(00)00153-1. [DOI] [PubMed] [Google Scholar]
- Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- Fava M, Borus JS, Alpert JE, et al. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154(3):426–428. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- Gaysina D, Cohen S, Craddock N, Farmer A, Hoda F, Korszun A, Owen MJ, Craig IW, McGuffin P. No Association With the 5,10-Methylenetetrahydrofolate Reductase Gene and Major Depressive Disorder: Results of the Depression Case Control (DeCC) Study and a Meta-Analysis. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:699–706. doi: 10.1002/ajmg.b.30665. [DOI] [PubMed] [Google Scholar]
- Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. Journal of epidemiology and community health. 2007;61:631. doi: 10.1136/jech.2006.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. American journal of epidemiology. 2007;165:1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- Hawley CJ, Gale TM, Sivakumaran T. Defining remission by cutoff score on the MADRS: selecting the optimal value. Journal of Affective Disorders. 2002;2:177–184. doi: 10.1016/s0165-0327(01)00451-7. [DOI] [PubMed] [Google Scholar]
- Iso H, Moriyama Y, Yoshino K, Sasaki S, Ishihara J, Tsugane S. Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey for the JPHC Study to assess folate, vitamin B6 and B12 intake: comparison with dietary records and blood level. J Epidemiol. 2003;13(1 Suppl):S98–101. doi: 10.2188/jea.13.1sup_98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B(6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr. 2008;62(8):939–45. doi: 10.1038/sj.ejcn.1602804. [DOI] [PubMed] [Google Scholar]
- Kelly CB, McDonnell AP, Johnston TG, Mulholland C, Cooper SJ, McMaster D, Evans A, Whitehead AS. The MTHFR C677T polymorphism is associated with depressive episodes in patients from Northern Ireland. J Psychopharmacol. 2004;18(4):567–71. doi: 10.1177/0269881104047285. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008;192:268–74. doi: 10.1192/bjp.bp.107.039511. [DOI] [PubMed] [Google Scholar]
- Lancot KL, Rapoport MH, Chan F, Rajaram RD, Strauss J, Sicard T, McCullagh S, Feinstein A, Kiss A, Kennedy JL, Bassett AS, Herrmann N. Genetic predictors of response to treatment with citalopram in depression secondary to traumatic brain injury. Brain Inj. 2010;24(7–8):959–969. doi: 10.3109/02699051003789229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Lawlor DA, Smith GD, Araya R, Timpson N, Day INM, Ebrahim S. The thermolabile variant of MTHFR is associated with depression in the British Women’s Heart and Health Study and a meta-analysis. Molecular Psychiatry. 2006;11:352–360. doi: 10.1038/sj.mp.4001790. [DOI] [PubMed] [Google Scholar]
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- Lievers K, Boers G, Verhoef P, Heijer M, Kluijtmans L, Put N, Trijbels F, Blom H. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine and cardiovascular risk. J Mol Med. 2001;79:522–528. doi: 10.1007/s001090100253. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, Ethanol, and Genotype Effects on Human Midbrain Serotonin Transporter Binding Sites and mRNA Levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and Folate Status in the US Population. Psychother Psychosom. 2003;72(2):80–87. doi: 10.1159/000068692. [DOI] [PubMed] [Google Scholar]
- Morrison K, Papapetriou C, Hol FA. Susceptibility to spina bifida; an association study of five candidate genes. Annals of Human Genetics. 1998;62(pt 5):379–396. doi: 10.1046/j.1469-1809.1998.6250379.x. [DOI] [PubMed] [Google Scholar]
- Olteanu H, Banerjee R. Human methionine synthase reductase, a soluble P-450 reductase-like dual flavoprotein, is sufficient for NADPH-dependent methionine synthase activation. Journal of Biological Chemistry. 2001;276(38):35558. doi: 10.1074/jbc.M103707200. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Lebowitz BD, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Alpert JE, Rosenbaum JF, Fava M. The relationship between serum folate, vitamin B12, and homocysteine levels in major depressive disorder and the timing of improvement with fluoxetine. Internation Journal of Neuropsychopharmacology. 2005;8:523–528. doi: 10.1017/S1461145705005195. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum Folate, Vitamin B12, and Homocysteine in Major Depressive Disorder, Part 1: Predictors of Clinical Response in Fluoxetine-Resistant Depression. J Clin Psychiatry. 2004;65:1090–1095. doi: 10.4088/jcp.v65n0810. [DOI] [PubMed] [Google Scholar]
- Paul TP, McDonnell AP, Kelly CB. Folic acid: neurochemistry, metabolism and relationship to depression. Human Psychopharmacol Clin Exp. 2004;19:477–488. doi: 10.1002/hup.614. [DOI] [PubMed] [Google Scholar]
- Payne ME, Hybels CF, Bales CW, Steffens DC. Vascular nutritional correlates of late-life depression. Am J Geriatr Psychiatry. 2006;14:787–795. doi: 10.1097/01.JGP.0000203168.28872.21. [DOI] [PubMed] [Google Scholar]
- Payne Martha E, Jamerson Brenda D, Potocky Christopher F, Ashley-Koch Allison E, Speer Marcy C, Steffens David C. Natural Food Folate and Late-Life epression. Journal of Nutrition For the Elderly. 2009;28:4, 348–358. doi: 10.1080/01639360903417181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330–5. doi: 10.3945/ajcn.2010.29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Byrum CE, McQuoid DL, Greenberg DL, Payne ME, Blichington TF, MacFall JR, Krishan KR. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Krishnan KR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacol Bull. 2002;36:58–68. [PubMed] [Google Scholar]
- Signorello LB, Buchowski MS, Cai Q, Munro HM, Hargreaves MK, Blot WJ. Biochemical validation of food frequency questionnaire-estimated carotenoid, alpha-tocopherol, and folate intakes among African Americans and non-Hispanic Whites in the Southern Community Cohort Study. Am J Epidemiol. 2010;171(4):488–497. doi: 10.1093/aje/kwp402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntossh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- Trivedi M, Rush JA, Wisniewski S. Evaluation of Outcomes with Citalopram for Depression using Measurement Based Care in STAR*D: Implications for Clinical Practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Penna Della, Noel PH, Lin Eh, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Vailas LI, Nitzke SA. Screening for risk of malnutrition in Wisconsin’s elderly. Wisconsin Medical Journal. 1995;94:495–9. [PubMed] [Google Scholar]
- Vaughn JD, Bailey LB, Shelnutt KP, von-Castel KM, Maneuval DR, Davis SR, Quinivan EP, Gergory JF, Theriaque DW, Kauwell GP. Methionine synthase reductase 66A-> G polymorphism is associated with increased plasma homocysteine concentration when combined with the homozygous methylenetetrahydrofolate reductase 677C-> T variant. The Journal of nutrition. 2004;134:2985–2990. doi: 10.1093/jn/134.11.2985. [DOI] [PubMed] [Google Scholar]
- Yang QH, Botto LD, Gallagher M, Friedman JM, Sanders CL, Koonts D, Nikolova S, Erickson JD, Steinberg K. Prevalence and effects of gene-gene and gene-nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: findings from the third National Health and Nutrition Examination Survey DNA Bank. The American journal of clinical nutrition. 2008;88(1):232–46. doi: 10.1093/ajcn/88.1.232. [DOI] [PubMed] [Google Scholar]
- Zaykin D, Zhivotovsky L, Weir BS. Exact tests for association between alleles at arbitrary numbers of loci. Genetica. 1995;96:169–178. doi: 10.1007/BF01441162. [DOI] [PubMed] [Google Scholar]
- Zeng W, Liu L, Tong Y, Liu HM, Dai L, Mao M. A66G and C524T polymorphisms of the methionine synthase reductase gene are associated with congenital heart defects in the Chinese Han population. Genet Mot Res. 2011;10(4):2597–2605. doi: 10.4238/2011.October.25.7. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wicker NJ, Shaw GM, Lammer EJ, Hendricks K, Suarez L, Canfield M, Finnell RH. Homocysteine remethylation enzyme polymorphisms and increased risks for neural tube defects. Mol Genet Metab. 2003;78:216–221. doi: 10.1016/s1096-7192(03)00008-8. [DOI] [PubMed] [Google Scholar]