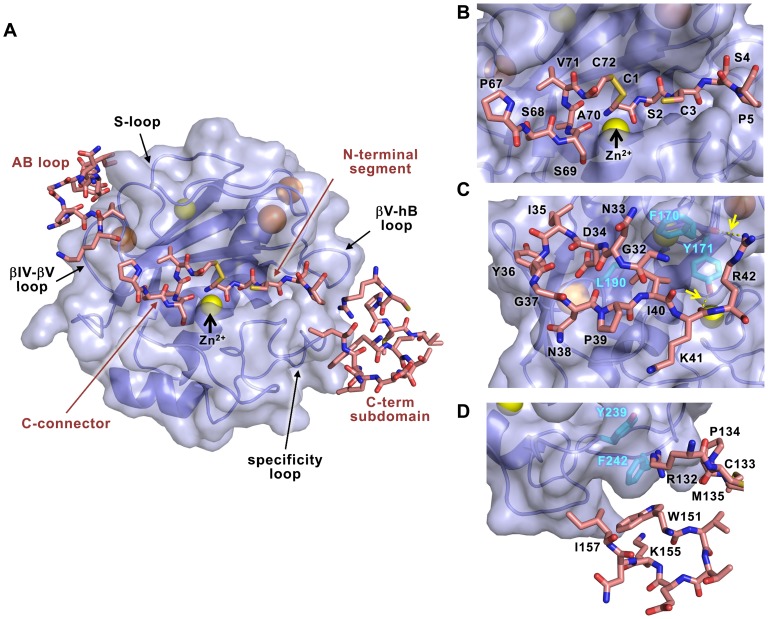
Figure 3. Contacts at the MMP-10cd/TIMP-2 interface.
MMP-10cd is rendered as a cartoon covered by semitransparent surface (slate) in the standard frontal orientation, with horizontally aligned TIMP-2 segments in stick representation (salmon). (A) Overview shows contacts of the TIMP-2 C-connector and N-terminal segment with MMP-10 substrate binding cleft (center), TIMP-2 AB loop contacts with MMP-10 S-loop and βIV-βV loop (upper left), and TIMP-2 C-terminal domain contacts with MMP-10 specificity and βV-hB loops (lower right). (B) Closer view of MMP-10 substrate binding cleft shows TIMP-2 C-connector residues occupying nonprimed subsites to the left of the catalytic zinc, while TIMP-2 N-terminal residues occupy primed subsites to the right of the zinc. (C) Closer view of the AB loop interactions reveals two interfacial H-bonds (dotted yellow lines highlighted by yellow arrows), and burial of the Ile-40 side chain in a hydrophobic pocket formed by MMP-10 residues Phe-170, Tyr-171, and Leu190. (D) GH loop residues 132–135 and multiple turn loop residues 151–157 on the C-terminal domain of TIMP-2 form minimal interactions with the MMP-10cd, including ring-stacking and cation-π interactions with MMP-10 specificity loop residues Phe-242 and Tyr-239.