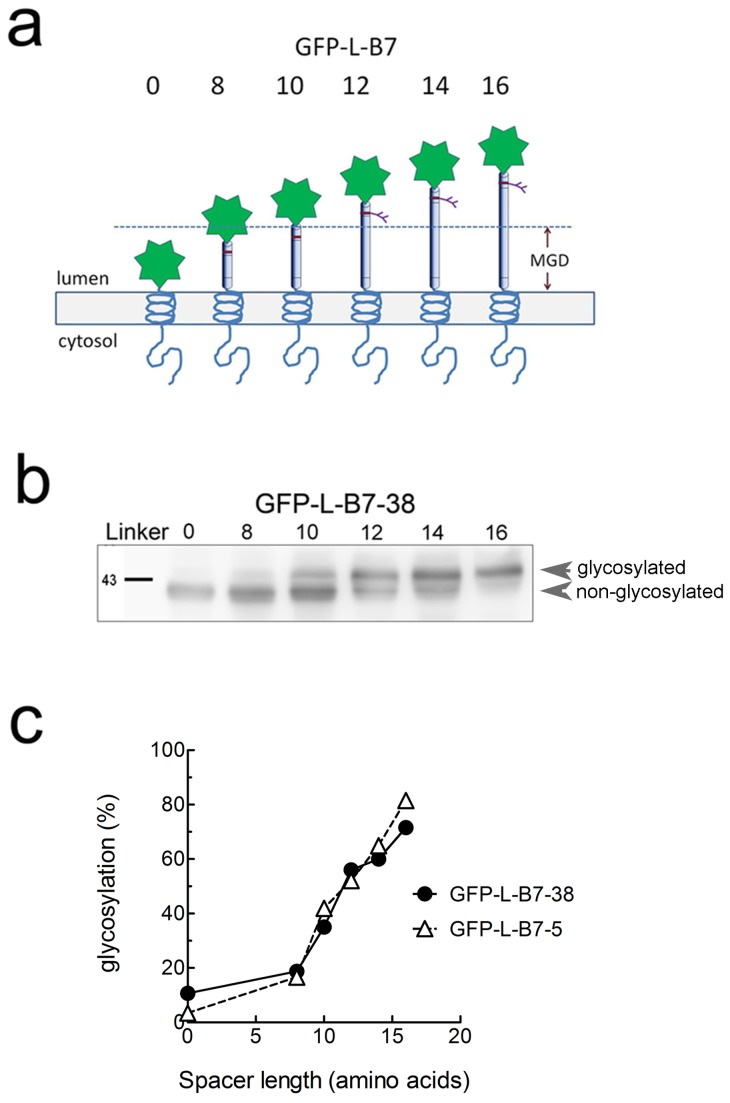
Figure 9. N-glycosylation mapping of chimeric proteins.
a) A series of GFP chimeric proteins with a full length cytoplasmic tail (GFP-L-B7-38) or a truncated cytoplasmic tail (GFP-L-B7-5) were generated with a single N-linked glycosylation sites engineered at variable distances (indicated by the number of amino acids in the linker domain, L) from the ER luminal membrane. Accessibility of enzymes responsible for glycosylation provides a measure the distance of the glycosylation site from the ER lumen membrane, and indirectly provides a measure of the position of the transmembrane domain in the ER membrane. b) 3T3 cells were transiently transfected with GFP-0-B7-38, GFP-8-B7-38, GFP-10-B7-38, GFP-12-B7-38, GFP-14-B7-38 or GFP-16-B7-38, harvested after 48 h, boiled in SDS PAGE buffer, separated on a SDS PAGE and immunoblotted with anti-HA antibody to visualize GFP chimeric proteins. Results show the glycosylated (upper band) versus non-glycosylated (lower band) forms of GFP-L-B7-38 with the indicated number of amino acids between the TM and N-liked glycosylation site. c) The amount of chimeric protein that is glycosylated as a percentage of total chimeric protein is shown versus spacer length (number of amino acids between the TM and glycosylation site) for GFP chimeric proteins with the original B7 cytoplasmic domain (GFP-L-B7-38) or a truncated B7 cytoplasmic domain (GFP-L-B7-5). Both chimeric proteins displayed similar dependence on linker length for glycosylation, indicating similar orientation and position of their transmembrane domains in the ER lumen.