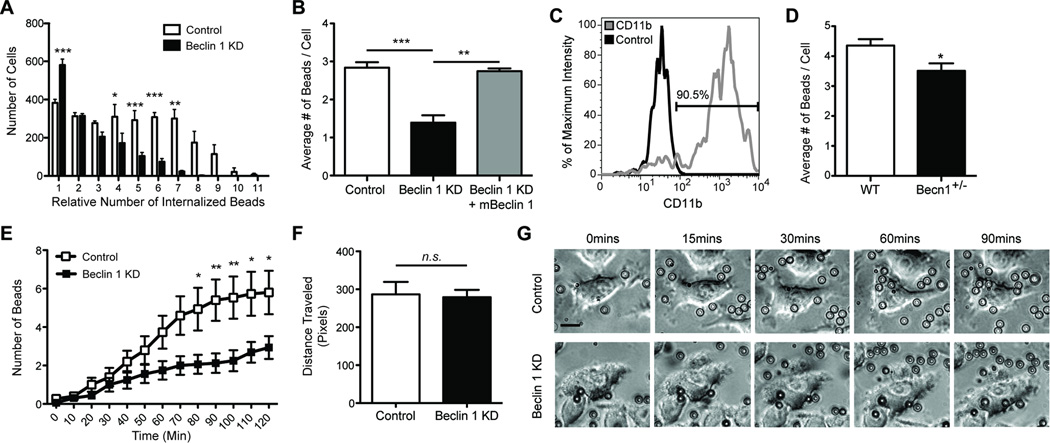
Figure 2.
Reduced beclin 1 impairs microglial phagocytic efficiency. BV2 microglia cells were infected with lentivirus encoding for luciferase shRNA (control) or beclin 1 shRNA (KD). (A) Phagocytosis of fluorescent latex beads was assayed by flow cytometry and analysis of flow cytometry histograms determined the number of BV2 cells phagocytosing relative quantities of beads. (B) Recovering beclin 1 levels with a lentivirus encoding for mouse beclin 1 (mBeclin 1) was sufficient to rescue phagocytic efficiency as measured by flow cytometry. (C) Primary microglia were isolated from beclin 1+/− (Becn1+/−) or wildtype (WT) mice and labeled with fluorescently tagged antibodies against CD11b or isotype control antibodies. The percent of CD11b+ cells in the microglial population was determined by flow cytometry relative to the cells receiving isotype control antibody. The microglial population was determined to be ~90% pure. (D) The average number of beads phagocytosed by Becn1+/− and WT primary microglia was quantified by flow cytometry. (E) Using live-cell imaging the number of beads phagocytosed over time was monitored. (F) Cell motility was also monitored by live-cell imaging. (G) Shown are representative images from live-cell imaging at various times after the administration of beads. To better visualize the beads an outline is provided around each bead. Scale bar, 15 µm. For further analysis of bead uptake please see Figure S2. Results were compared by a two-way ANOVA with a Bonferroni post-test (A,E), a one-way ANOVA with a Tukey’s post-test (B), or an unpaired Student’s t test (D,F) and are representative of at least 3 independent experiments (A–B; n=3 per group, D; n=4–6 per group, E–F; n=10–16 per group). Values are mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001.