Table 1.
Scope of Electron-deficient Heterocycles in Hydrosilylation - Dehydrogenative Cyclizationa
 | ||||
|---|---|---|---|---|
| entry | intermediate | 2, yieldb | product | 3, yieldb |
 |
 |
|||
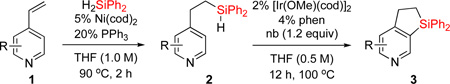
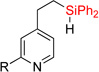
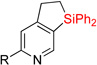
| 1 | 2a: R = H | 84% | 3a: R = H | 83% |
| 2c | 2a: R = H | 83% | 3a: R = H | 74–82% |
| 3 | 2b: R = Me | 86% | 3b: R = Me | 87% |
| 4 | 2c: R = F | 86% | 3c: R = F | 68% (5:1) |
| 5 | 2d: R = Cl | 61% | 3d: R = Cl | 64% |
 |
 |
|||
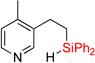
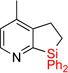
| 6 | 2e | 95% | 3e | traces |
 |
 |
|||
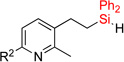
| 7 | 2f: R = H | 93% | 3f: R = H | 79% |
| 8 | 2g: R = Me | 90% | 3g: R = Me | 86% |
| 9 | 2h: R = Cl | 83% | 3h: R = Cl | 75% |
 |
 |
|||
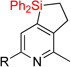
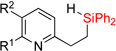
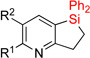
| 10 | 2i: R1 = H, R2 = H | dec. | 3i: R1 = H, R2 = H | N/A |
| 11 | 2j: R1 = Me, R2 = H | 48%1 | 3j: R1 = Me, R2 = H | 36% |
| 12 | 2k: R1 = Me, R2 = F | 76%d | 3k: R1 = Me, R2 = F | 73% |
| 13 | 2l: R1 = OMe, R2 = H | 67% | 3l: R1 = OMe, R2 = H | 72% |
| 14 | 2m: R1 = CF3, R2 = H | 76% | 3m: R1 = CF3, R2 = H | 77% |
 |
 |
|||
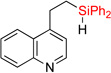
| 15 | 2n | 97% | 3n | 74% |
 |
||||
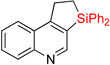
| 16 | 2o | 96% | 3o | 78% |
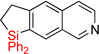 |
||||
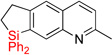
| 17 | 2p | 90% | 3p | 74%e |
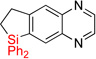 |
||||
| 18 | 2q | 94% | 3q | 56%e |
Hydrosilylation reaction conditions: 1 (1.0 mmol), H2SiPh2 (1.02 mmol), Ni(cod)2 (5 mol %), PPh3 (20 mol %), and THF (1.0 mL) were stirred at 90 °C for 2 h under nitrogen. Dehydrogenative coupling reaction conditions: 2 (0.5 mmol), [Ir(cod)OMe]2 (2 mol %) and 1,10-phenantroline (4 mol %), norbornene (1,2 equiv), and THF (1.0 mL) were stirred at 100 °C for 12 h under nitrogen.
Isolated yield.
Reaction was performed on 10 mmol scale using 2.5 mol % Ni-catalyst and 0.25–0.5 mol % Ir-catalyst.
10 mol % Ni(cod)2 was used.
48 h at 100 °C.
