Abstract
Purpose
We evaluated the biomechanical strength of two all suture anchors (ASA) of reduced diameter (1.4 mm) and compared them with the standard screw anchor (SA) with larger diameter (5.5 mm) used in rotator cuff tears.
Methods
We conducted 30 uniaxial vertical pullout tests using Material Testing System Instron 5566A until failure of the anchorage defined as rupture of the threads or anchor or detachment of the anchor. Anchor fixation was on tuberosities of fresh bovine humerus bone. ASAs were spaced four millimetres apart and were compared with a control SA implanted on the same greater tubercle at two centimetres. The tests were all performed at room temperature in a dry environment. Tensile loads (10 mm/min) were applied parallel to the axis of insertion. A preloading of 10 N was used to overcome loading artifacts of the test sample at the beginning of the test.
Results
Student’s t test showed no statistically significant difference between anchors in terms of load to failure (ASA: force 265.06 ± 87.25 N versus SA : 325.35 ± 113.46 N; p = 0.09) and mean elongation at rupture (ASA : 23 ± 7 mm versus SA : 21 ± 6 mm; p = 0.46).
Conclusions
In vitro, this experimental study showed no statistically significant difference in pullout strength and displacement between ASA and SA at a chosen level of significance (p < 0.05).
Introduction
Rotator cuff repair by tendon reinsertion on the proximal humerus aims to treat pain and initial functional impairment and prevent the occurrence of eccentric shoulder osteoarthritis [1–5]. Due to the development of minimally invasive techniques (miniopen or arthroscopic), anchors have gradually replaced transosseous sutures, which have been the gold standard for cuff surgery [6, 7]. Tendon reinsertion is now usually done with suture threads mounted on anchors to restore attachment to bone. These metal, absorbable or polyetheretherketone (PEEK) anchors, screwed or impacted, are considered the reference device for tendon reinsertion [8, 9]. Not all anchors have the same pullout strength or thread resistance, however [8, 9]. Anchors require surgical preparation of the bone insertion site (drilling or punching), and their size (up to 6.5 mm) limits the number of anchors on the insertion site, which can lead to bone loss and result in imprecise tendon reinsertion. Finally, interarticular migration (of the entire anchor or fragments), prominent intra-articular portions and resorption granuloma fractures have been described in anchor failures [10–14]. Due to the volume and reported complications of anchors, some surgeons prefer to repair the rotator cuff with transosseus points, allowing a very small gap between attachment points without using anchors [15].
Recently, all suture anchors (ASAs) made with textile and with a reduced diameter were introduced for arthroscopic repair of the glenoid labrum. These anchors are entirely composed of suture and require a bone tunnel of 1.4 mm in diameter, which is substantially smaller than that of the screw anchor (SA) (three to six millimetres). In tendon-insertion repair of rotator cuff muscles, the use of small-diameter ASAs (1.4 mm) could increase the number of bone anchors compared with a rigid SA of larger diameter while preventing complications theoretically related to the use of a rigid anchor. There is no study in the literature evaluating the biomechanical pullout resistance of the ASA in rotator cuff repair.
The hypothesis of our biomechanical study is that the pullout strength and displacement of two ASAs of reduced diameter (1.4 mm) is statistically equivalent to that of a single SA with a larger diameter (5.5 mm) (control).
Methods
The ASA has a reduced diameter of 1.4 mm and does not use a rigid fixation device. During an insertion repair of one or more rotator cuff tendons, the reduced diameter can theoretically increase the number of anchorage points, thus providing a larger number of anchors compared with anchors of a greater diameter. To provide an answer to our hypothesis, we conducted a biomechanical, in vitro experimental study using bovine bone with uniaxial tensile test measuring pullout strength of two ASAs compared with a control SA. (Fig. 1).
Fig. 1.

Screw anchor (SA) 5.5 mm, all suture anchor (ASA) 1.4 mm
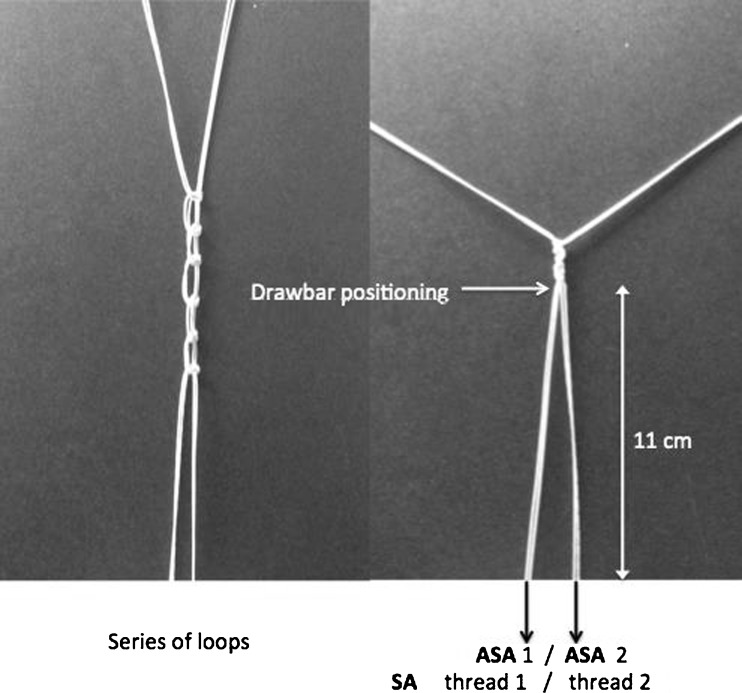
Fifteen fresh bovine tuberosities of the humerus bone were stripped of all soft tissue, and one area of insertion was defined: a cancellous trough created by decortication with an electrically powered burr on footprint cortex. The bullocks were all between two and three years of age. Bovine humerus was chosen for several reasons: reproducibility, ease of machining and local availability. We paid particular attention to limit cycles of freezing and thawing during exposure to room temperature, which may alter the biomechanical properties of bone; even five freezing–thawing cycles do not significantly affect the biomechanical properties of bovine cancellous bone [16]. In order to increase the repeatability of our experiment, biomechanical tests were performed the same day, making necessary advanced preparation of bovine bone. In total, only two cycles of freezing–thawing were needed: one to allow preparation of the tuberosities; the other for testing. Exposure time at room temperature was under 30 minutes for the preparation and under 12 hours for testing. Bovine greater tubercles were placed in the same position (greater tubercle to the apex) and then set in resin blocks (Axson Technologies ®, F23 Fascast polyurethane resin) of 130 × 180 × 70 mm to ensure their immobility during trials. An experimental assembly specially manufactured for the study was designed to hold every substrate on the test platform. Biomet (Warsaw, IN, USA) provided 30 commercially available Juggerknot ASAs (1.4 mm) in which was inserted one thread of polyethylene of ultrahigh molecular weight (MaxBraid thread size 1). Stryker company (San Jose, CA, USA) provided 15 commercially available SAs (PEEK ZipTM 5.5 mm) in PEEK with two independent eyelets in which two polyethylene threads of ultra-high molecular weight (FiberForce 2 threads, size 2) were inserted. A control PEEK anchor was chosen because it is considered a reference standard [9] in cuff repair and is an alternative to metal (nonbiodegradable plastic radiotransparent, high mechanical resistance to deformation) [17]. Fixation of anchors was conducted in accordance with manufacturers’ recommendations using the arthroscopic ancillary dedicated to each anchor. The anchors were introduced perpendicular to the tangent through the implanted bone surface. The ASAs were implanted at four millimetre intervals. To limit the variability of bone support, each pair of ASAs was compared with a control SA implanted on the same tuberosity at two centimetres from the ASAs. The thread of each anchor was tied to the thread of the second ASA by a series of loops. The anchoring threads were placed such that they do not cross before the knot on the drawbar (Fig. 2). For the SAs, the two threads were knotted by a series of loops identical to that of the ASAs. All threads were tied on the drawbar arbitrarily at 11 cm from the surface of the implanted bone with an identical tension on each thread (Fig. 2). This device was designed to prevent unequal thread length between anchor types during the pullout test.
Fig. 2.
Drawbar positioning
We conducted a uniaxial pullout test at the speed of 10 mm/min using a Material Testing System Instron 5566A until failure of the anchorage, defined as rupture of threads, rupture or anchor pullout (Fig. 3). The tests were all performed at room temperature in a dry environment. Tensile load was applied parallel to the axis of insertion. A preloading of 10 N was used to overcome loading artifacts of the test sample at the beginning of test. Each experimental condition was repeated 15 times to assess repeatability. Parameters measured were load to failure (breaking force in Newtons), elongation at rupture (mm) and reason for anchor failure.
Fig. 3.
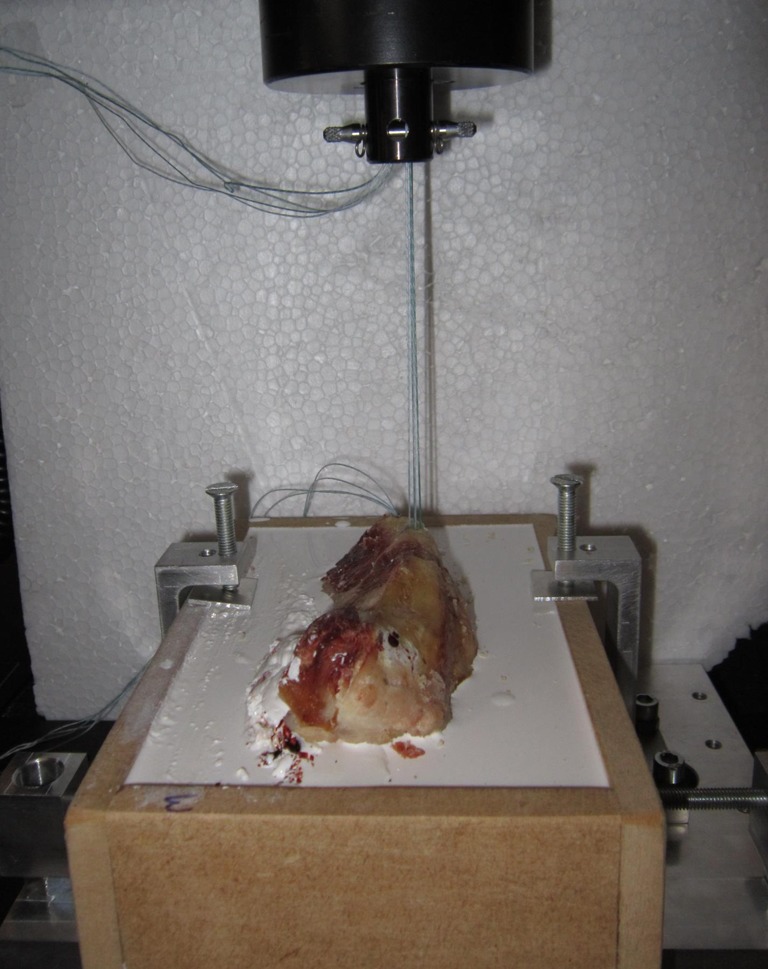
Pullout test: vertical strength
Statistical analysis was performed using the software GraphPad Prism 6. Test data were analysed for means and standard deviation (SD). Kolmogorov–Smirnov tests were used to confirm that all distributions were normal Unpaired Student’s t test was used to compare the average load to failure and displacement between ASA and SA group. Significance was set at p ≤ 0.05.
Results
During testing, two test samples were not tested because the implantation was not consistent with the experimental protocol (anchor damage during implantation). The reason for ending the test in the ASA group was anchor pullout from supports in 12 cases (Fig. 4) and thread rupture in one case. In the SA group, the reason for ending the test was anchor fracture (eyelet) in five cases (Fig. 5) and anchor pullout from support in eight cases (Table 1). The average load to failure for the ASA group was 265.06 ± 87.25 N and 325.35 ± 113.46 N for SA. The average displacement (mm) measured at rupture was 23 ± 7 mm for ASA and 21 ± 6 mm for SA (Table 2).
Fig. 4.
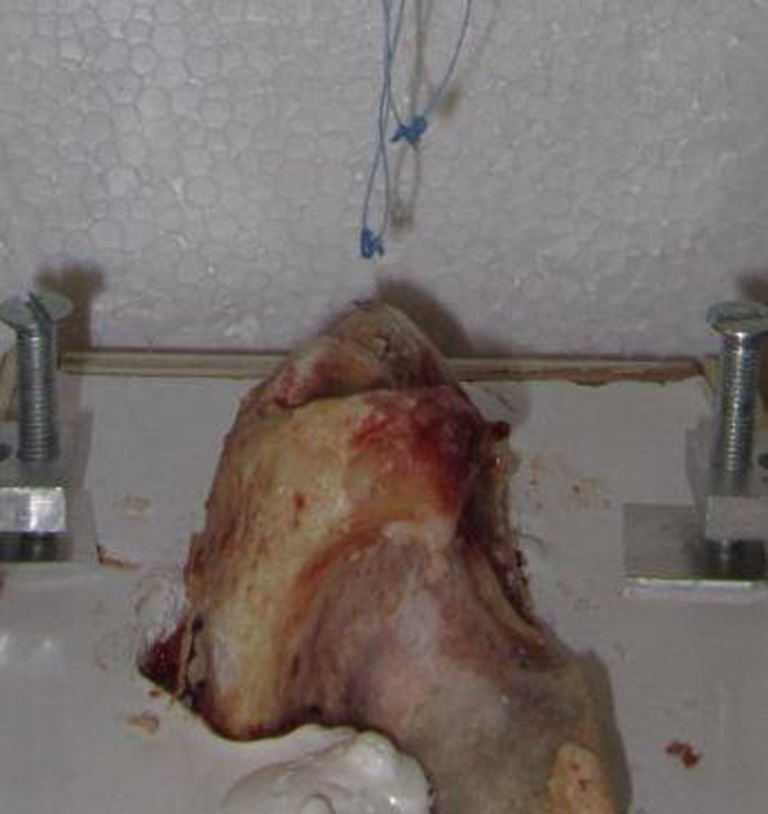
All suture anchor (ASA) failure: anchor detachment
Fig. 5.
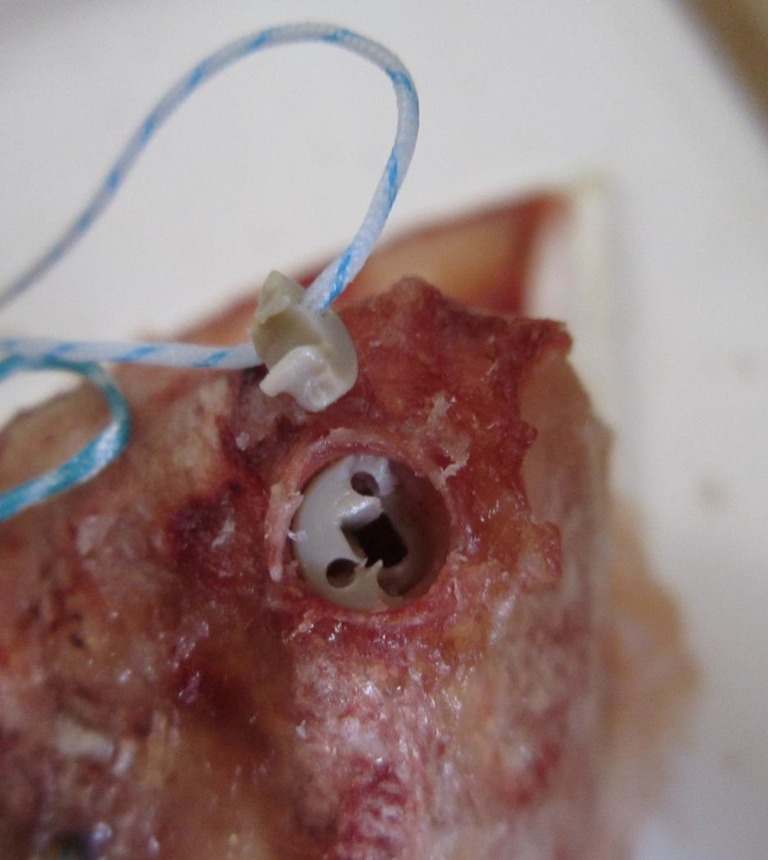
Screw anchor (SA) failure: eyelet fracture
Table 1.
Reasons for failure for each anchor type
| Anchor pullout | Eyelet fracture | Thread rupture | |
|---|---|---|---|
| Screw anchor (SA) 5.5 mm | 8 | 5 | 0 |
| All suture anchor (ASA) 1.4 mm (x2) | 12 | 0 | 1 |
Table 2.
Average pullout strength and displacement for all suture anchor (ASA) and screw anchor (SA)
| Average rupture strength (newton) | Standard deviation | Average displacement (mm) | Standard deviation | |
|---|---|---|---|---|
| ASA 1.4 mm (x2) | 265.06 | ±87.25 | 23 | ±7 |
| SA 5.5 mm | 325.35 | ±113.46 | 21 | ±6 |
| P value | 0.09 | 0.46 |
Analysis of traction-force curves
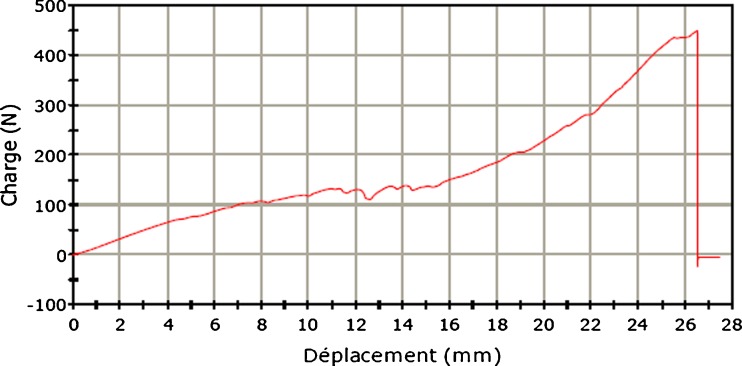
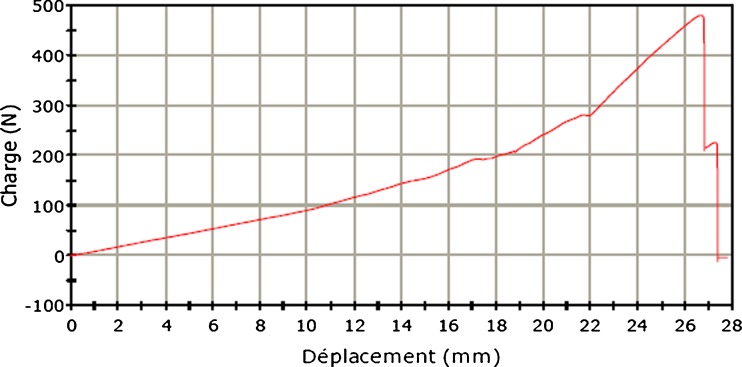
Analysis of traction-force curve (load) in the displacement function showed a long elastic phase, a short plastic phase and a sudden return to the lack of load related to detachment or rupture in both anchorage systems. All curves for the ASA during elastic deformation (150 ± 27 N) showed a plateau phase corresponding to displacement of the test sample of an average of 3 mm. (Figs. 6 and 7).
Fig. 6.
Traction curve of all suture anchor (ASA)
Fig. 7.
Traction curve of screw anchor (SA)
Statistical analysis
Student’s t test showed no statistically significant difference between the ASA and control SA in terms of pullout force (p = 0.099) and mean displacement at rupture (p = 0.459). (Table 2)
Discussion
This study was not designed to compare the pullout strength of anchors available on the market but to determine the pullout strength and displacement of an experimental device using two ASAs of 1.4 mm placed four millimetres apart compared with one SA control anchor of 5.5 mm. We postulated that there was no statistically significant difference between pullout strength and displacement of a double-fixed bone ASA and a single-fixed control SA. Our experimental study showed no statistically significant difference between the two types of anchor at the chosen level of significance (p <0.05) measured on different criteria (average load to failure and displacement). In vitro, the use of multiple ASAs increased overall anchorage strength, with an average breaking strength measured in our study of 265.06 ± 87.25 N. The reasons for ASA failure were anchor detachment and detachment or anchor fracture at the eyelet level for SAs.
A previous biomechanical study [9] showed that ASAs used individually underwent lower pullout strength than traditional SAs or impacted ones. The reasons for anchor failure were different (for ASA, anchor detachment; for SAs, anchor detachment and fracture) [6, 18, 19]. The pullout strength obtained in that study was lower, but this is probably related to the nature of the support (bovine tuberosity in our study; porcine femoral diaphysis in the Barber et al. [9, 17] studies). During SA failure (anchor detachment or eyelet rupture), the release of intra-articular foreign bodies (anchor fragments) can have adverse effects, especially on cartilage [10–14]. For ASAs, it is likely that the complications of failure are mild and comparable with those of a transosseous suture. Analysis of traction curves showed a slightly different behavior of ASAs compared with control anchors, which were not described in previous studies [8, 9]. Indeed, after an average threshold of 150 ± 27 N, anchorage mobilises slightly to reach the subcortical bone (Fig. 6). This mobilisation corresponds to the plateau phase of the tension curve of the ASA; 3 mm of displacement probably corresponds with less tissue approximation and ASA compression . This finding emphasises the importance of a rigorous implantation and the value of a manual preload performed by the surgeon after implantation in order to place it as close as possible under the subcortical bone. Finally, the average displacement measured at the breakpoint corresponds to the displacement of the entire test sample (support, anchor, thread). The amount of displacement measured for the two anchorage points is mainly due to stretch deformation of the suture loop and does not reflect anchor migration, which can be found in vivo. This, therefore, does not permit a conclusion regarding the importance of anchor movement, especially for ASAs, during the plateau phase.
In the literature, the optimal technique described for rotator cuff repair combines a high load to failure and tendon compression after suture, allowing tendon healing [20]. Medial positioning at the edge of the cartilage guarantees a better bone stock, which is required for optimal anchor attachment [21]. Its association with a double-row repair leads to better biomechanical strength (greater pullout strength in axial tension) compared to a single-row repair, prevents formation of a gap between the bone and tendon and provides better coverage and increased tendon compression on the “foot print” [21–23]. Furthermore, in vitro, increasing the number of sutures leads to decreased gap formation, undercycling loading and increased load to failure [24]. Optimal fixation of a repaired rotator cuff requires the use of a larger number of anchors and increases the risk of propagation fracture between anchors. Moreover, during a standard repair, anchoring can be limited to a single point because of the large diameter of the anchor and the small size of the lesion. Application of bone–tendon could then be limited by the formation of tendon plication. In order to improve the anatomy and isometry when transfixing a rotator cuff tear, the reduced anchor diameter allows an increased number of anchorage points and a more uniform application of tendon–bone at the edge of the cartilage. This provides sufficient pullout strength without the complications described for rigid anchors.
Ours was an in vitro study in a dry environment at room temperature, which is its main limitation, and thus correlation is difficult with physiological biomechanics of the shoulder joint. To obtain perfect reproducibility of the experimental conditions and to achieve better comparability with other biomechanical studies, we conducted a vertical pullout test measuring load to failure of two anchorage, not an evaluation of a cyclic traction force on anchors or the link between rotator cuff tendon and suture anchor placement. Traction force was applied perpendicular to the bovine bone surface, which is very different in vivo, where the angle of tendon-force application with respect to the bone surface is more acute, variable according to the position of the shoulder and depends on the type of repair (single or double row). However, application of vertical tension force prevents thread ruptures at the eyelet and potentiates detachment resistance of SAs compared with angular tension tests [25, 26]. Applying the pullout test at one angle only did not allow us to determine ASA behaviour during a pullout test at a greater angle and thus its absolute detachment resistance in vivo. However, because when using the ASA there is no eyelet, angular tension-force variation may have less impact on detachment resistance.
We have found no published biomechanical study providing a threshold of in vivo or in vitro minimum resistance detachment to ensure successful repair. The average maximum force developed by the supraspinatus muscle on the healthy greater tuberosity is more important in active abduction and is estimated by Wallace et al. to be 300 N [27]. Some authors use this estimated limit as an objective to ensure efficiency of their repair [28]. It is likely that the minimum resistance to detachment that will allow rotator cuff healing is multifactorial (bone and tendon trophicity, muscle involution), and is probably less when inserted in the degenerative rotator cuff.
The mechanical approach to cuff repair described should not be used exclusively on all patients and must involve a biological approach. Indeed, the biological properties of bone, tendon and muscle trophicity probably play a role in the success of rotator cuff repair. Increasingly, authors are studying the influence of reported parameters in relation to successful rotator cuff repair, such as the use of growth factors [29] or compression of the tendon–bone repair [22]. It is clear that consideration of the mechanical technique and enhancement of the healing process will favour successful repair.
Conclusions
In vitro, the experimental model suggests that two ASAs have equivalent pullout strength as that of an SA. This biomechanical study is a preliminary experiment in the use of small-diameter ASAs in rotator cuff repair as well as a clinical study evaluating the efficacy of this repair.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Di Schino M, Augereau B, Nich C (2012) Does open repair of anterosuperior rotator cuff tear prevent muscular atrophy and fatty infiltration? Clin Orthop Relat Res. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 2.Cofield RH. Rotator cuff disease of the shoulder. J Bone Joint Surg Am. 1985;67:974–979. [PubMed] [Google Scholar]
- 3.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;304:43–53. [PubMed] [Google Scholar]
- 4.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 5.Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89:1533–1541. doi: 10.2106/JBJS.F.00305. [DOI] [PubMed] [Google Scholar]
- 6.Pietschmann MF, Frohlich V, Ficklscherer A, Hausdorf J, Utzschneider S, Jansson V, Muller PE. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2008;16:504–510. doi: 10.1007/s00167-007-0460-3. [DOI] [PubMed] [Google Scholar]
- 7.Craft DV, Moseley JB, Cawley PW, Noble PC. Fixation strength of rotator cuff repairs with suture anchors and the transosseous suture technique. J Shoulder Elbow Surg. 1996;5:32–40. doi: 10.1016/S1058-2746(96)80028-0. [DOI] [PubMed] [Google Scholar]
- 8.Mazzocca AD, Chowaniec D, Cote MP, Fierra J, Apostolakos J, Nowak M, Arciero RA, Beitzel K. Biomechanical evaluation of classic solid and novel all soft suture anchors for glenoid labral repair. Arthroscopy. 2012;28(5):642–648. doi: 10.1016/j.arthro.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Barber FA, Herbert MA, Hapa O, Rapley JH, Barber CA, Bynum JA, Hrnack SA. Biomechanical analysis of pullout strengths of rotator cuff and glenoid anchors: 2011 update. Arthroscopy. 2011;27(7):895–905. doi: 10.1016/j.arthro.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan A, Ghodadra N, Karas V, Salata MJ, Cole BJ. Complications of bioabsorbable suture anchors in the shoulder. Am J Sports Med. 2012;40(6):1424–1430. doi: 10.1177/0363546511417573. [DOI] [PubMed] [Google Scholar]
- 11.Park AY, Hatch JD. Proximal humerus osteolysis after revision rotator cuff repair with bioabsorbable suture anchors. Am J Orthop. 2011;40(3):139–141. [PubMed] [Google Scholar]
- 12.Goeminne S, Debeer P. Delayed migration of a metal suture anchor into the glenohumeral joint. Acta Orthop Belg. 2010;76(6):834–837. [PubMed] [Google Scholar]
- 13.Dunn JC, Friedman DJ, Higgins LD. Anchor fracture leading to supraspinatus failure. J Shoulder Elbow Surg. 2010;19(1):e24–e26. doi: 10.1016/j.jse.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee S, Weiser L, Connell D, Wallace AL. Glenoid rim fracture in contact athletes with absorbable suture anchor reconstruction. Arthroscopy. 2009;25(5):560–562. doi: 10.1016/j.arthro.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad CS, Stewart AM, Izquierdo R, Bigliani LU. Tendon-bone interface motion in trans osseous suture and suture anchor rotator cuff repair techniques. Am J Sports Med. 2005;33:1667–1671. doi: 10.1177/0363546505278252. [DOI] [PubMed] [Google Scholar]
- 16.Kang Q, An YH, Friedman RJ. Effects of multiple freezing-thawing cycles on ultimate indentation load and stiffness of bovine cancellous bone. Am J Vet Res. 1997;58(10):1171–1173. [PubMed] [Google Scholar]
- 17.Barber FA, Herbert MA, Beavis RC, Barrera OF. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859–867. doi: 10.1016/j.arthro.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Dejong ES, DeBerardino TM, Brooks DE, Judson K. In vivo comparison of a metal versus a biodegradable suture anchor. Arthroscopy. 2004;20:511–516. doi: 10.1016/j.arthro.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Barber FA, Cawley P, Prudich JF. Suture anchor failure strength - an in vivo study. Arthroscopy. 1993;9(6):647–652. doi: 10.1016/S0749-8063(05)80500-6. [DOI] [PubMed] [Google Scholar]
- 20.Andres BM, Lam PH, Murell GAC, Phil D. Tension, abduction and surgical technique affect foot print compression after rotator cuff repair in an ovine model. J Shoulder Elbow Surg. 2010;19:1018–1027. doi: 10.1016/j.jse.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff C, Braunstein V, Milz S, Sprecher CM, Fischer F, Tami A, Ahrens P, Imhoff AB, Hinterwimmer S. Assessment of bone quality within the tuberosities of the osteoporotic humeral head: relevance for anchor positioning in rotator cuff repair. Am J Sports Med. 2010;38(3):564–569. doi: 10.1177/0363546509354989. [DOI] [PubMed] [Google Scholar]
- 22.Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403–410. doi: 10.2106/JBJS.D.02887. [DOI] [PubMed] [Google Scholar]
- 23.Wall BJ, Keener JD, Brophy R. Double-row vs single-row rotator cuff repair: a review of the biomechanical evidence. J Shoulder Elbow Surg. 2009;18:933–941. doi: 10.1016/j.jse.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Jost PW, Khair MM, Chen DX, Wright TM, Kelly AM, Rodeo SA. Suture number determines strength of rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):e100. doi: 10.2106/JBJS.K.00117. [DOI] [PubMed] [Google Scholar]
- 25.Bardana DD, Burks RT, West JR, Greis PE. The effect of suture anchor design and orientation on suture abrasion: an in vitro study. Arthroscopy. 2003;19(3):274–281. doi: 10.1053/jars.2003.50032. [DOI] [PubMed] [Google Scholar]
- 26.Deakin M, Stubbs D, Bruce W, Goldberg J, Gillies RM, Walsh WR. Suture strength and angle of load application in a suture anchor eyelet. Arthroscopy. 2005;21(12):1447–1451. doi: 10.1016/j.arthro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Rossouw DJ, McElroy BJ, Amis AA, Emery RJ. A biomechanical evaluation of suture anchors in repair of the rotator cuff. J Bone Joint Surg Br. 1997;79(3):458–461. doi: 10.1302/0301-620X.79B3.6983. [DOI] [PubMed] [Google Scholar]
- 28.Sward L, Hughes JS, Amis A, Wallace WA. The strength of surgical repairs of the rotator cuff. A biomechanical study on cadavers. J Bone Joint Surg Br. 1992;74(4):585–588. doi: 10.1302/0301-620X.74B4.1624521. [DOI] [PubMed] [Google Scholar]
- 29.Schaer M, Schober M, Berger S, Boileau P, Zumstein MA. Biologically based strategies to augment rotator cuff tears. Int J Shoulder Surg. 2012;6(2):51–60. doi: 10.4103/0973-6042.96995. [DOI] [PMC free article] [PubMed] [Google Scholar]