Figure 5.
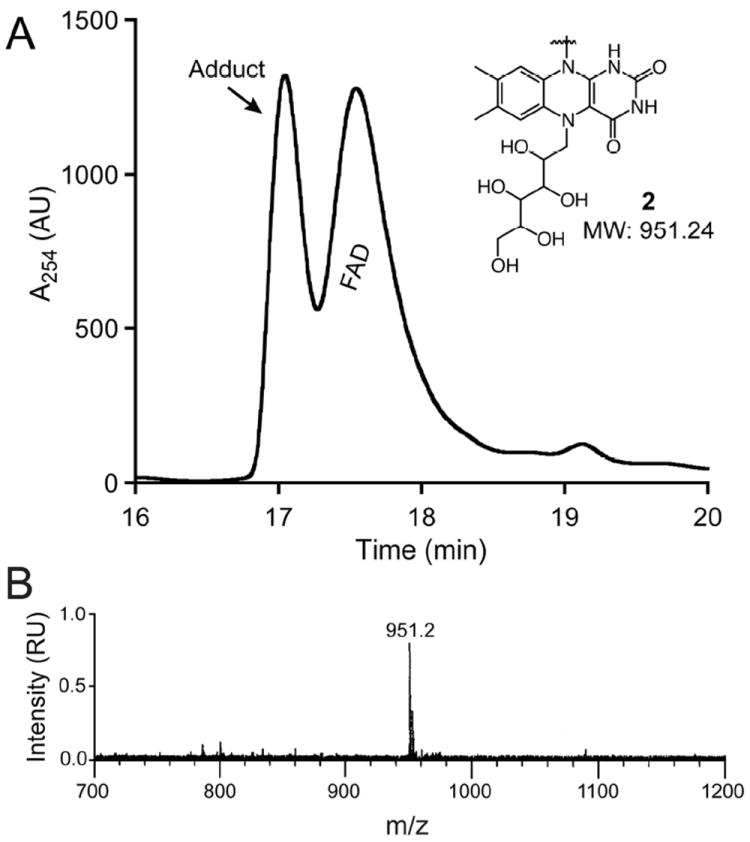
Proposed structure and active site of CeUGM. (A) CeUGM homology model (green) generated using SWISS-MODEL superimposed with the structure of M. tuberculosis UGM (PDB Code: 1V0J; wheat). (B) A comparison of residues in the active site. Select conserved residues predicted (CeUGM) or known (M. tuberculosis UGM) involved in substrate binding are highlighted, with C. elegans residue numbers denoted first. (C) Relative activity of wild-type, the R187A variant, and the R336A variant CeUGM at a UDP-Galf concentration of approximately 12-fold above the Km of the wild-type enzyme. Error bars represent the standard deviation of triplicate measurements. Relative activity is derived from normalizing to wild-type enzyme.
